
The Inflation Reduction Act’s limit on Medicare Part D spending can lead to savings for patients prescribed oral chemotherapy drugs.

The Inflation Reduction Act’s limit on Medicare Part D spending can lead to savings for patients prescribed oral chemotherapy drugs.

The rule places new requirements on plans, including making sure prior authorization and other utilization management tools for mental health are not stricter than those used for medical benefits.

This analysis finds that 57.4 million adults under the age of 65 could potentially be eligible for GLP-1 drugs based on currently approved FDA indications, including 36.2 million people with obesity.
Digital technology, AI and other tools will allow for many specialty infusion therapies to be administered in ambulatory centers, allowing payers to customize programs.
The Type 1 Diabetes Grand Challenge has provided awards to six universities —including three in the United States — to fund research of novel therapies to treat patients with type 1 diabetes.

The private-label biosimilar will be available at an 80% discount off Stelara. Evernorth has not released information about which company will be producing the biosimilar.

Technology is advancing to allow the prior authorization process to operate behind the scenes through electronic systems that are integrated with health plan information.

Pharmacy benefit managers aren't getting mentioned on the campaign trail, but there are some signs Congress could enact reform legislation after the Nov. 5 election.

Changes to the methodology for how the Health Equity Index is assessed could see Medicare Advantage Star ratings — and payments to plans — drop.

Joseph Shields of TransparencyRx predicts there will be more lawsuits against self-funded employers alleging they’ve mismanaged prescription drug benefits through PBMs.

During an interview, Joseph Shields of TransparencyRx discusses the PBM legislation that is expected to move forward in Congress in the next few months.

Outcomes-based agreements can take away clinical uncertainties, so payers have a guarantee that they're not spending money on something that’s not going to work.

A new study finds that diabetes and mental health are linked. People with chronic diabetes complications are at a three-times greater risk of having a mental health condition.

The new program provides large employers with access to Capital Rx’s technology platform for claim adjudication, invoicing, and reimbursement.

MedImpact is not requiring patients currently taking Humira to switch, and all three products are on a preferred tier.

Through the PfizerForAll platform, patients can connect with a healthcare professional, find and book vaccines, order tests, and find medication savings information.
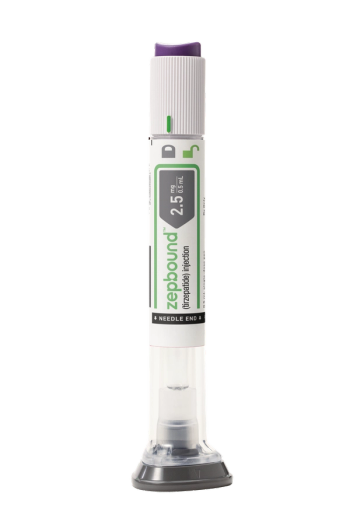
The monthly price of the 2.5 mg Zepbound single-dose vial is $399 and the 5 mg dose is $549, which Lilly officials said is in line with the savings program for without insurance.

Beginning in 2025, Express Scripts will favor biosimilars of Humira, including its own private-label version.
The updated vaccines include the KP.2 strain of the Omicron variant, which is believed to be contributing to the increases in COVID-19 infections this summer.

Avalere’s Kylie Stengel talks about the regional shifts in formularies and utilization management in Medicare Part D prescription drug plans.

Researchers in a new study found that the overall risk of RSV-related hospitalization for adults with inflammatory bowel disease was greatest for those who were 18 to 49 years of age and those who were 65 years of age or older.

The Medicare program is expected to see some aggregate savings from the negotiated drug prices. Beneficiaries, because of out-of-pocket caps next year, may not see the benefit, that is until the next round or two of negotiations if CMS choses drugs that are not heavily rebated.

The negotiated prices range from 38% to 79% discounts off of list prices, according to the Department of Health and Human Services.

New research looks at the impact of state-level caps on out-of-pocket costs for insulin. Before the caps, most commercial enrollees were already paying costs below the cap amounts.

The KFF analysis reported on the number of prior authorizations by plans, but found the data do not contain enough information to assess prior authorization by type of service, type of plan or reasons for denials.

Prademagene zamikeracel is a cell therapy in development to treat patients with recessive dystrophic epidermolysis bullosa (RDEB), a rare connective tissue disorder.

Merck and Johnson & Johnson have both acquired bispecific antibodies with applications in autoimmune and inflammatory conditions.

No information, however, is available about which insurance plans are providing coverage of Anktiva or what the utilization management requirements are.

New analysis shows that differences in the methods used to set drug payment amounts under Part B versus under Part D result in different payment amounts for the same drugs.

In a recent survey, payers said their priorities include addressing the impacts of Medicare’s Drug Price Negotiation Program and the Part D redesign.