Articles by Denise Myshko

Total revenue for the company increased for the second quarter of 2024, but CVS Health is facing increased medical cost trend in its Medicare/Medicaid health plans and lowered revenue in its pharmacy benefit business. As a result, CVS Health has lowed its outlook for the year and parted ways with its Aetna president.

After less than a year, Aetna president Brian Kane is out at CVS Health, it was announced today at an investor call. The change was made to address the continued pressure in the company’s healthcare benefits business.
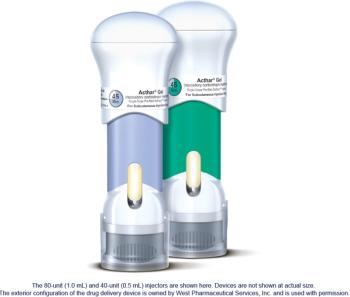
SelfJect is priced at parity with the Acthar Gel multi-dose vial and syringe.
Driving this growth will be the approvals of novel biologics, such as gene therapy, RNA-based therapeutics, and antibody-drug conjugates.
Medicaid children with type 1 diabetes are three times more likely to receive metformin than commercial enrollees even though metformin is only indicated for use in patients with type 2 diabetes.

Cigna officials cite significant growth in Evernorth Health Services and Express Scripts for the increase in revenue.

As many as 40% of people with type 1 diabetes are unaware that they have the disease.

Suzetrigine, a new type of pain medication, has a Prescription Drug User Fee Act target action date of Jan. 30, 2025, for patients with acute pain.

CVS Caremark is requiring step therapy through a generic prescription proton pump inhibitor before providing coverage.

Higher costs per claim, coupled with an increased number of patients using specialty drugs, has contributed to higher spend.

Keri Althoff, Ph.D., discusses how a model developed at Johns Hopkins can assess the comorbidities that patients with HIV may face by 2030.

Improving equity for people with HIV requires health leaders to recognize that patients have different risk factors, Keri Althoff, Ph.D., of Johns Hopkins says.
A substudy of the FLAIR phase 3 trial found that participants favored intramuscular injection of Cabenuva over the subcutaneous version due to pain and the development of nodules and reddening of the skin.

There is still much to be done to build on the progress already made in HIV and AIDS, panelists said during a session at the International AIDS Conference.

Results from the DYAD trial reinforce a previous study that found Dovato is not inferior for patients with undetectable viral load who switched from Biktarvy
The FDA has required all approved CAR T therapies to have a boxed warning about the risk of secondary malignancies after treatment.

Teleophthalmology enables earlier access to eye care for patients with diabetes, but vulnerable populations often have worse insurance coverage.
The stem cell transplant donor had only one copy of the gene that prevents HIV from entering cells, which opens up the possibility of a cure through gene therapy.
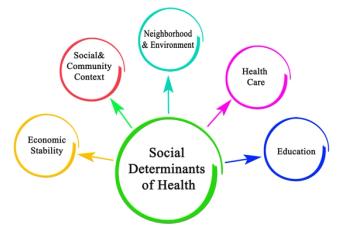
Economic instability and other social determinant of health can impact whether people with diabetes seek out healthcare for their eyes, putting them at risk for vision loss.

Dr. Charles C. Wykoff discusses how a one-time gene therapy could benefit patients with wet age-related macular degeneration.

Dr. Charles C. Wykoff discusses interim results from a phase 2 trial of a gene therapy to treat with patients with wet age-related macular degeneration.

UnitedHealth is still feeling the impact of the Change Healthcare cyberattack.

The switch to biosimilars helped Navitus clients offset increased utilization in the non-specialty category and the introduction of higher-cost specialty drugs.
The FDA is asking for information about the manufacturing process, as well as the type 1 diabetes indication.

The FTC’s interim report on pharmacy benefit managers (PBMs) was just the latest effort to highlight what some say is an industry that profits at the expense of patients and independent pharmacists. The PBMs say the report paints an incomplete, misleading picture. Others say it shows the FTC is prepping its antitrust case.

The PBM industry said the FTC has not been objective, and that efforts to limit PBM negotiating tools would put patients at the mercy of drug manufacturers.

Findings from observational studies in the past have not been consistent on the link between type 1 diabetes and epilepsy.
Company officials have said the bundled payment program could negatively impact sales of Xphozah, which was approved last year to reduce serum phosphorus in patients with kidney disease on dialysis.

Donanemab — now with the brand name of Kisunla — slows cognitive and functional decline by up to 35% and has a list price of $695.65 per vial.

A new guidance aims to provide a consensus on autoantibody testing and monitoring to identify those with early-stage type 1 diabetes whose disease could progress.




























