
Researchers say patient-reported disability can be used as a metric in outcomes-based contracts related to the care of multiple sclerosis patients.


Researchers say patient-reported disability can be used as a metric in outcomes-based contracts related to the care of multiple sclerosis patients.
Drugmakers had hoped that inhibiting receptor-interacting protein kinase would decrease inflammation and reduce neuronal degeneration in patients with multiple sclerosis and other neurodegenerative conditions
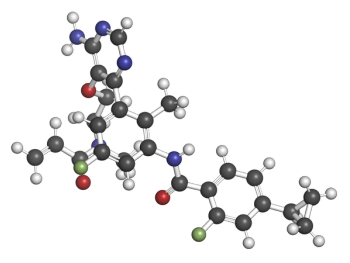
But three — tolebrutinib, fenebrutinib, and remibrutinib — are now in late-stage trials.
One of the projects involves,studying a type of cholesterol that may promote myelin repair.

Segesterone acetate is a laboratory-made derivative of progesterone, a sex hormone used in many hormonal contraceptives

Roche's drug is one of a number competing to become the first Bruton's kinase inhibitors approved as a treatment for multiple sclerosis.
A positive result for Bruton’s tyrosine kinase inhibitor after the drug had been put on partial clinical hold due to concerns about liver toxicity.

Research has observed a decreased risk of autoimmune disorders, including MS, in people who consume alcohol. However, it is not known whether patients may reduce their alcohol consumption as their disease progresses.

In a recent study, the investigational drug, PIPE-307, effectively induced OPC differentiation and remyelination in MS donor human brain tissue and an animal model and restored lost function in an MS mouse model.
Intranasal administration is a novel delivery method for the treatment of inflammatory human diseases.

New research published in Nature Scientific Reports revealed that people with MS are more prone to experience a short-term reduction in disability and brain lesion volume after receiving stem cell therapy.
A new study out of Germany, published in Science Translational Medicine in June, looked at peripheral blood mononuclear cells and serum collected from two independent cohorts of patients with MS to identify three endophenotypes of the disease.

From the cohort study, 67 participants developed Multiple Sclerosis after enrollment.
Researchers completed a phase 2 clinical trial investigating the use of autologous stem cell-derived neural progenitors as a treatment option in patients with progressive forms of multiple sclerosis.

TG Therapeutics, Briumvi’s manufacturer, currently recommends that patients who can get pregnant use an effective method of contraception during treatment with Briumvi and for at least six months after the last Briumvi dose. A pregnancy test before each treatment is also recommended.

Progentos will use the funding to advance its multiple sclerosis (MS) program through human proof of concept clinical studies and expand its small molecule pipeline to other degenerative conditions.

This data is based off of a study conducted by a group of researchers led by Niklas Frahm from the German Multiple Sclerosis Registry to compare the characteristics of patients with MS who switched from their first disease-modifying therapies (DMT) with those of patients who continued taking their first DMT.
Although several clinical trials have confirmed the safety and efficacy of rituximab in oncology and autoimmune disease, and even as off-label use in MS, real-world evidence is still necessary to help guide clinicians and managed care professionals in their treatment and coverage decision-making.


Drug repurposing has recently emerged as an attractive pathway for developing new treatments due to its relatively fast and cost-efficient trajectory. Because obesity and MS share inflammatory properties, researchers used data from the FDA Adverse Event Reporting System to investigate the association between weight loss-inducing drugs and MS

Darina Georgieva, Pharm.D., and her colleagues from the department of pharmaceutical services at Vanderbilt University Medical Center, conducted a retrospective observational study to learn the costs avoided through specialty pharmacist interventions for patients at the Vanderbilt MS Clinic. The study results were published in the Journal of Managed Care and Specialty Pharmacy earlier this month.
Long-term data from the phase 3 DAYBREAK trial affirmed sustained efficacy of ozanimod for relapsing forms of multiple sclerosis, with a high amount of patients who were relapse-free at 6 years.

Women are at risk for disease progression when they stop treatment for multiple sclerosis during pregnancy.

As with adult-onset MS, early treatment of pediatric-onset MS (POMS) is essential to halt disease progression, including early disability, brain volume loss, and cognitive impairment.