
In 2019, the worldwide burden of HCV exceeded 58 million people, with 14.9 million infections occurring in women aged 15 to 49 years.

In 2019, the worldwide burden of HCV exceeded 58 million people, with 14.9 million infections occurring in women aged 15 to 49 years.
Just over half (51%) of patients who treated with the experimental agent, called elafibranor, achieved a biochemical response compared with only 4% of those patients randomly assigned to placebo.
The FDA has set March 24, 2024, as PDUFA date for the drug.

Researchers at the University of California, San Francisco, report positive results from the No One Waits study.
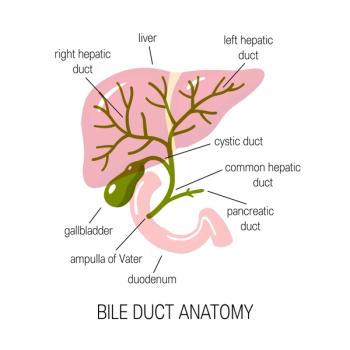
Capsid assembly modulators (CAM) work by assembling flawed hepatitis B capsids and preventing the formation of new infectious viruses.

New results demonstrate seladelpar can reduce alkaline phosphatase and itching in patients with primary biliary cholangitis, a chronic liver disease.
Findings from a retrospective cohort analysis revealed patients with alcohol-associated liver disease diagnosed with and prescribed at least one medication had a decreased risk of mortality.
The drug has been engineered using a proprietary glycopegylated technology that is designed to increase its overall half-life.
Gilead’s hepatitis D antiviral, bulevirtide, was dealt a setback last year when the FDA issued a complete response letter. Positive results from a phase 3 trial of bulevirtide, were reported in July.

To add precision to the nomenclature and nosology and remove stigma, experts are moving to rename nonalcoholic fatty liver disease as metabolic dysfunction-associated steatotic liver disease.

Assembly Bio’s established areas of research is focused on herpesviruses (HSV), hepatitis B virus and hepatitis D virus.
Based on the results published in the New England Journal of Medicine, the FDA has granted breakthrough status to the FGF21 analogue. The drug's maker is planning a phase 3 trial.

A new review and analysis suggests a combination treatment regimen for children in acute liver failure due to autoimmune hepatitis may help them maintain native liver survival.

The impact of alcohol use on SVR is less certain as some payers and clinicians have different guidelines as far as alcohol abstinence and receiving DAA therapy.

Researchers of a study investigated the association between health-related physical fitness and liver function indicators in hopes of finding potential preventative measures and personalized exercise prescriptions for those living with liver complications.
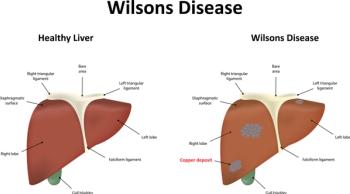
Two gene therapy trials using adeno-associated viral vectors are underway. A review paper published recently in the New England Journal of Medicine says lifelong treatment with chelators is effective but adherence is a problem.
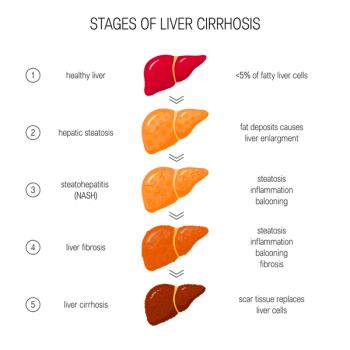
The FDA has granted priority review and assigned a Prescription Drug User Fee Act date of March 14, 2024, for resmetirom to treat adult patients with nonalcoholic steatohepatitis (NASH) who have liver fibrosis.
About 65% of adults in the United States drink sugar-sweetened beverages daily, which increases the risk for liver cancer and mortality in women.

The CDC reports that the incidence of hepatitis A and B has declined.

The advent of the direct-acting antivirals, such as Harvoni (ledipasvir and sofosbuvir) means people can be treated for HCV infection if they receive a heart from an HCV-viremic donor, according to a recent review paper. The supply of hearts available for transplantation has increased, partly because HCV-viremic individuals are now part of the donor pool.
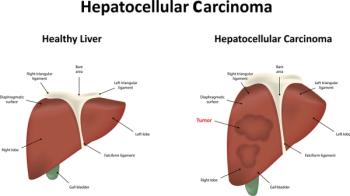
A retrospective study found that chronic hepatitis B-related hepatocellular carcinoma (HCC) patients with metabolic-associated fatty liver disease (MAFLD) had better outcomes than patients who did not have MAFLD.

Since the introduction of direct-acting antiviral therapies, which boast a 95% cure rate, the reported number of HCV cases has fallen.
That is the rate among those with health insurance. It is 1 in 6 among those without it. Prices have dropped but cost remains a barrier and there are other ones.