Results from a retrospective cohort study show lower all-cause mortality and fewer hospitalizations and intestinal complications among those treated with semaglutide, tirzepatide and other GLP-1s.


Results from a retrospective cohort study show lower all-cause mortality and fewer hospitalizations and intestinal complications among those treated with semaglutide, tirzepatide and other GLP-1s.

Research might produce treatments of involving transplants with a few, selected types of bacteria tailored to an individual's microbiome.

The results showed that 26% of patients who received tulisokibart achieved clinical remission at week 12 compared with 1% of those who received placebo.

Treatment with Entyvio (vedolizumab), an integrin receptor antagonist, followed by Humira (adalimumab), a tumor necrosis factor blocker, resulted in the lowest overall incidence of adverse events, according to an industry-sponsored study.

Results of a survey show that Black/Indigenous/People of Color or Hispanic patients with Crohn's disease or ulcerative colitis were more likely to report poor symptom control and greater difficulty accessing care from specialists and twice as likely to have an IBD-related emergency department visit compared with White non-Hispanic patients.

Study eases some of the concern about Crohn's disease and ulcerative colitis creating undue infection risk.

Tremfya, a monoclonal antibody that blocks IL-23, is also approved to treat patients with plaque psoriasis and active psoriatic arthritis.
Entyvio (vedolizumab) and Xeljanz (tofacitinib) are the most commonly studied therapies, according to the Pfizer-sponsored review, and when the two were compared, Xeljanz showed higher remission rates.
The emergence of biologics and other disease-modifying therapies for UC has changed the face of UC treatment in the last 20 years. However, although the number is decreasing, up to 15% of patients with UC will still require colectomy surgery during their lifetime.

According to a study, increased competition brought about by the emergence of biosimilars in the market has resulted in a CAGR decrease of 5% to 20% in biosimilar and reference product pricing. It is estimated that biosimilars have saved payers, and in the end patients, more than $100 billion over five years.

Researchers say depictions of inflammatory bowel disease (IBD). which includes ulcerative colitis, don't reflect the growing the incidence of IBD in older and nonwhite populations.

Previous studies on lipid levels and disease activity are inconsistent, and a causal relationship between lipid levels and IBD remains dubious.

Tremfya is an interleukin (IL)-23 and CD64-inhibiting monoclonal antibody. The FDA approved it in 2017 as a moderate to severe plaque psoriasis treatment. It has since been approved to treat psoriatic arthritis.
A study published in Nature Immunology focused on understanding the origins of stem-like T cells in ulcerative colitis patients. By analyzing colon tissue samples from human patients, researchers found a significant population of stem-like T cells in inflamed regions of the large intestine compared to healthy individuals.

In one key finding of the study, about half of the healthcare professionals listed bowel urgency as one of the top five symptoms that impact patients’ lives, however, only about a quarter of them ranked it as a top three symptom affecting treatment decisions.

In clinical guidelines, TNF inhibitors remain the preferred first-line advanced treatment for UC despite the emergence of new biologics approved for UC and Crohn’s disease. Head-to-head trials comparing the effectiveness of these treatments are scarce.

In a study recently published in BMJ Open Gastroenterology, researchers led by Pernille Dige Ovesen from the department of gastroenterology and hepatology in Copenhagen University Hospital investigated the effect of concomitant corticosteroid therapy on treatment outcomes in patients with UC initiating infliximab.
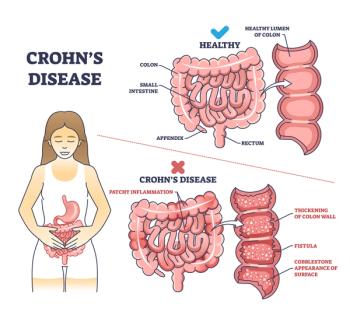
About 30% of people with Crohn’s disease, but on a population basis, it is rare event.

Home testing for fecal calprotectin, a commonly used marker in IBD diagnosis, and symptom quetionnaires might hasten the diagnosis of inflammatory bowel disease.

At 12 months, the rate of IBD-related surgeries for the monitored group was 1% versus 7% for the control group.

If the company' experimental agent is approved, it would join Humira (adalimumab), Rinvoq (upadacitinib) and Skyrizi (risankizumab) in AbbVie's portfolio.
Fecal microbiota transplant has not been approved for ulcerative colitis, but off-label use is a possiblity.

Insurance policy adherence to clinical guidelines ranged from 6% to 59%, according to findings reported in American Journal of Gastroenterology.

Elimination of insurance-mandated step therapy and allowing the Medicare beneficiaries to use copay assistance programs are among the changes the American Gastroenterological Association (AGA) wants to see.