
Two posters presented at the European Respiratory Society International Congress found use of Dupixent improved lung function by 12 weeks with sustained responses.

Two posters presented at the European Respiratory Society International Congress found use of Dupixent improved lung function by 12 weeks with sustained responses.


Findings presented at the European Respiratory Society found that using Dupixent to treat acute exacerbations of chronic obstructive pulmonary disease (COPD) reduced duration of systemic corticosteroid days.

Patients with atopic dermatitis (AD) and caregivers of children with AD highlighted strategies that they felt would manage AD-related mental health burden.
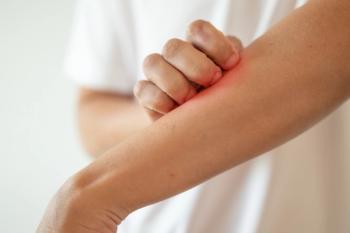
Chronic prurigo is a chronic itchiness can set in motion an itching -scratching cycle that results in scarring. A case series at a hospital in Zurich, Switzerland, shows Dupixent (dupilumab) injections are an effective treatment.

A post hoc analysis of two phase 3 trials identified that as many as 95% of patients with chronic rhinosinusitis with nasal polyps (CRSwNP) had type 2 inflammatory signatures depending on the definition of inflammation used.

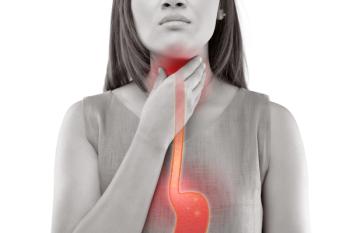
Most (91%) of the patients were seen by gastroenterologists. Those seen by allergists were more likely to have comorbid atopic conditions, such as asthma, allergic rhinitis and atopic dermatitis.

Patients with severe asthma with or without coexisting chronic rhinosinusitis and nasal polyps (CRSwNP) experienced continued improvements in exacerbations and lung function in an extension study.
Patients with eosinophilic esophagitis experience a range of symptoms that can vary by age, which may contribute to younger patients experiencing a delay in diagnosis, according to a poster presented at the American College of Allergy, Asthma and Immunology Annual Scientific Meeting.

Adherence to follow-up examinations is crucial for early identification of recurrence of chronic rhinosinusitis with nasal polyps (CRSwNP).

Despite differing characteristics, patients have similar responses to treatment regardless of when they were diagnosed with eosinophilic esophagitis (EoE).

Symptoms of chronic rhinosinusitis with nasal polyps can be a burden for patients, but Dupixent was able to successfully reduce the number of days with severe symptoms.

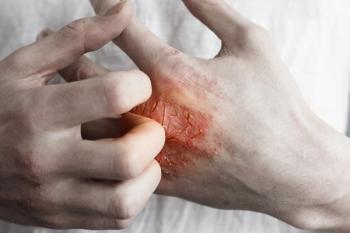
A real-world study of children with moderate-to-severe atopic dermatitis (AD) in China found Dupixent can reduce symptoms and improve pruritus. Traditional therapies had little effect, according to the researchers.

A sizable majority (69%) of eosinophilic esophagitis (EoE) patients only had one food trigger.

The majority of guidelines and recommendations available limit biologics to cases of severe and uncontrolled chronic rhinosinusitis with nasal polyps (CRSwNP) and require prior surgery.

Results reported at a meeting of the European Respiratory Society this week show a low rate of severe asthma attacks and sustained improvement in lung function among the children who participated in the yearlong add-on study.
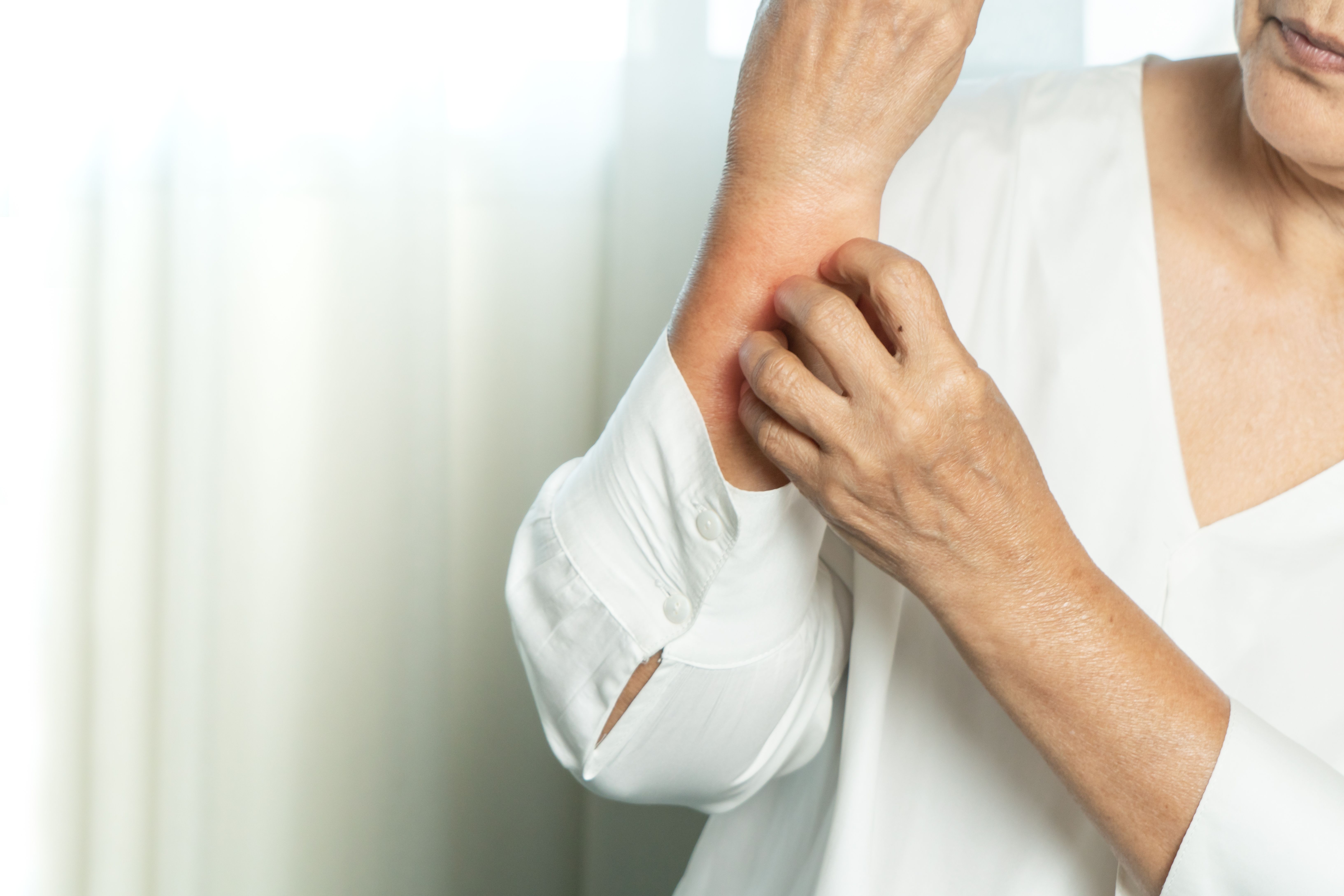
A national study in Germany identified the baseline characteristics of patients with moderate-to-severe atopic dermatitis (AD) to understand how they are usually treated with Dupixent in the real world.

Therapeutic goals of reducing eosinophil counts below a certain level in eosinophilic esophagitis (EoE) has a marginal benefit for the impact on quality of life and treatment burden.
Patients with persistently controlled atopic dermatitis (AD) who had their doses reduced were able to maintain their low disease activity.

Patients with chronic rhinosinusitis with nasal polyps had the greatest number of outpatient visits and the shortest time between visits.

Less-effective coping strategies, such as passive and palliative reaction, were associated with a significant impact on health-related quality of life in eosinophilic esophagitis (EoE).

The increases had no impact on the efficacy of the drug and were rarely associated with symptoms or sequelae.

Certain problems such as pain/discomfort, worrying and fear of the persistence/recurrence of atopic dermatitis (AD) became more common among patients with AD since the start of the COVID-19 pandemic.

Dupixent was already approved for patients 6 years and older. The new approval makes the drug available to children 6 months to 5 years whose atopic dermatitis is inadequately controlled.

A 52-week open-label extension study supported the long-term use of Dupixent to treat adolescents with moderate-to-severe atopic dermatitis.