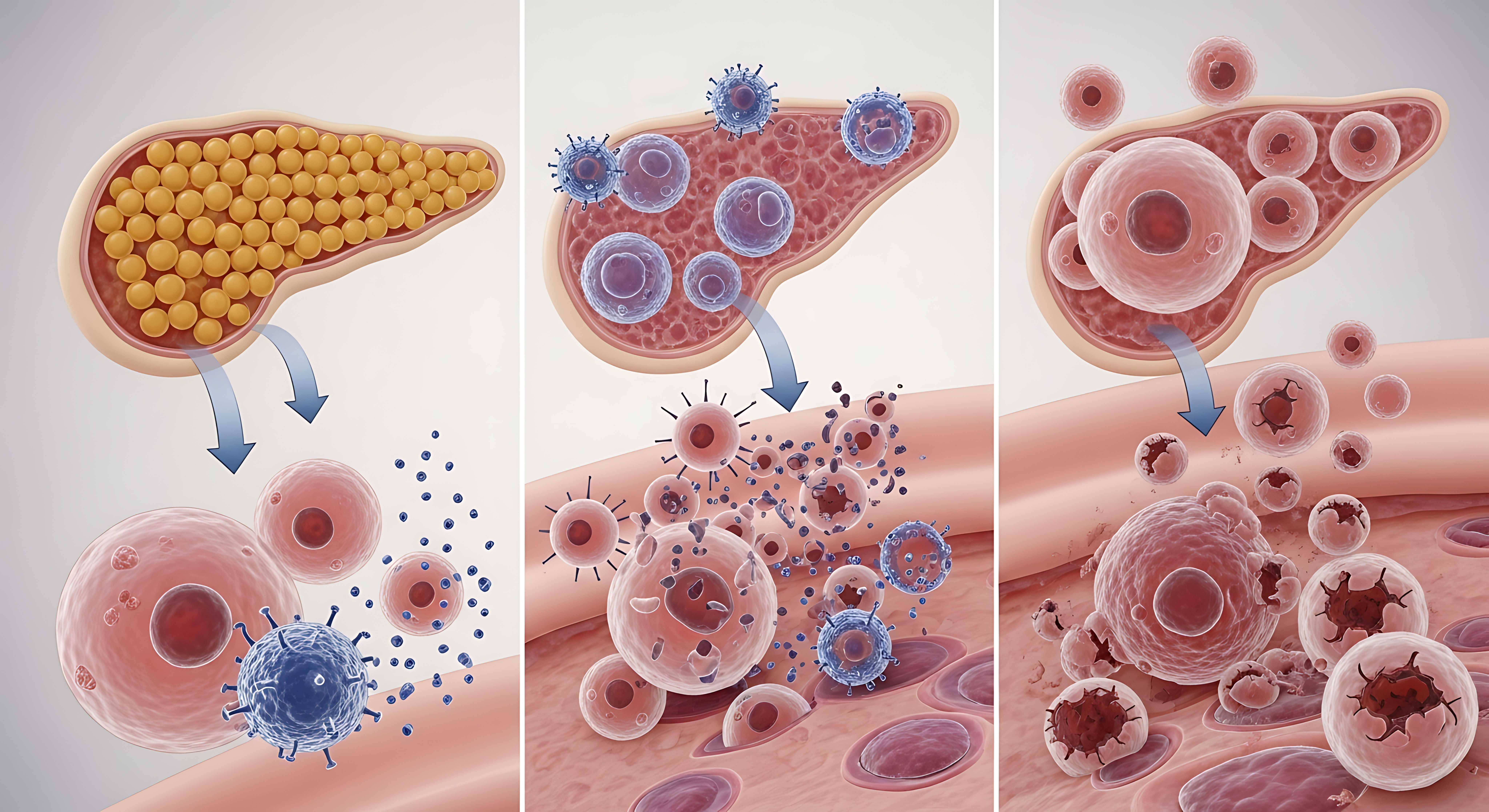
Type I Diabetes
Latest News
Latest Videos
CME Content
More News

University of Pennsylvania researchers say their findings could potentially lead to the development of a blood test to monitor people at high risk of developing Type 1 diabetes.

A new study suggests epigenetic changes at Type 1 diabetes susceptibility genes may explain the risk profile of children born of parents with the disease.

Diabetes self-management is associated with a significant burden and mental health impacts, but automated, ‘tubeless’ systems may be able to decrease that burden.

Type 1 diabetes, celiac disease, and Hashimoto thyroiditis are all strongly associated with HLA class II genes, authors of an observational study that found links among the diseases.

Patients with Type 1 diabetes are often excluded from diabetes and weight loss trials.
Tzield, a monoclonal antibody approved for stage 2 Type 1 diabetes, now seeks approval for stage 3 disease based on trial data showing that it slowed beta cell loss in children and adolescents.
Socioeconomic factors are a key risk factor for Type 2 diabetes, but a new analysis says the same is not true when it comes to Type 1 diabetes.
In a recent trial, verapamil showed a trend toward preserving insulin-producing beta cells in patients with Type 1 diabetes but failed to achieve statistical significance.
Investigators have identified the lowest dose of the immunosuppressive ATG needed to preserve beta cells in newly diagnosed children with Type 1 diabetes with the lowest side effects.
The NIH is funding a new study at Weill Cornell that aims to identify the regulatory roles of gene variants involved in Type 1 diabetes development.

Researchers suggest that access to treatment and socioeconomic resources can influence glycemic control and disparities in the management of patients with Type 1 diabetes.
Cadisegliatin acts in the liver to increase the activity of glucokinase, an enzyme that plays a role in regulating blood glucose levels.
Researchers say that a deficiency in the brain of the hormone leptin is as much a part of the cascade that can lead to diabetic ketoacidosis as a lack of insulin is. This opens the potential for the development of new therapies for patients with Type 1 diabetes that target the brain.
Farxiga, along with insulin and monitoring for diabetic ketoacidosis, shows promise for kidney and glucose control in youth with Type 1 diabetes.

In a study in mice, a wireless implant was able to release glucagon as an emergency treatment for hypoglycemia, a serious complication of Type 1 diabetes.

Barbie wears a continuous glucose monitor and an insulin pump and has a phone displaying a CGM app.
After a one-year follow-up, 10 of 12 patients given the islet cell therapy zimislecel no longer required insulin. Vertex officials said regulatory submissions are expected in 2026


Results presented at the 85th Scientific Sessions of the American Diabetes Association in Chicago highlight the potential for AI to detect disease before signs and symptoms occur.
A cloud-based digital twin simulation, coupled with automated insulin delivery, allows patients with diabetes to experiment with their own data on glycemic control in a safe simulation environment before adjusting their system.

The findings of a new study lend support for type 1 diabetes autoantibody screening in people with other autoimmune conditions to prevent complications such as diabetic ketoacidosis.

The risk of major cardiac events was 50% higher in adult-onset type 1 diabetes than in population controls, and the risk of death from diabetic coma or ketoacidosis was seven times as high as those with type 2 diabetes.

Led by the University of Queensland’s research facility, Frazer Institute, the trial examining the drug ASITI-201 is the first of its kind in the world and has already dosed its first five participants.

Researchers have found that Sotyktu, which inhibits the protein TYK2, protects insulin-producing beta cells and, at the same time, is able to reduce inflammation.

TIX100 is a small molecule inhibitor of TXNIP, a protein involved in cellular stress response and metabolism. This protein plays a role in the development and progression of diabetes.