Hemophilia
Latest News
Latest Videos

Podcasts
CME Content
More News

Switching to extended half-life from standard half-life therapies for hemophilia enhances treatment adherence and reduces infusion frequency, despite initial cost increases.

Out of the more than 800,000 people worldwide living with hemophilia, around 20% of those with hemophilia A and 3% with hemophilia B develop inhibitors, preventing their bodies from responding to standard clotting factor therapies.

A new study found that patients with hemophilia A could safely switch directly from emicizumab to Mim8 without a washout period, meaning they did not need to stop treatment before starting the new medication and could maintain continuous protection against bleeding.

It was found that those with severe hemophilia B have less than 1% of normal factor IX activity, often experiencing spontaneous bleeding that can cause joint damage or life-threatening events.


According to a new study in Haemophilia, up to 60% of patients with severe HA have no known family history, making early diagnosis more difficult.

Patients diagnosed with acquired (autoimmune) hemophilia, a serious and rare bleeding disorder, have high rates of hospital readmissions because of infections and bleeding, as well as high death rates especially among older patients.
Results of a real-world study that included people with hemophilia from 33 countries show that people with hemophilia B, especially those with inhibitors, have the greatest unmet need.

Qfitlia (fitusiran) is the first therapy for both hemophilia A or B, with or without inhibitors, available in the United States.

The context matters — and it is not a matter of semantics.

The FDA recently approved the treatment by Novo Nordisk for hemophilia A or B with inhibitors, designed to prevent or reduce bleeding episodes in patients aged 12 and older.
Hympavzi is the first anti-tissue factor pathway inhibitor approved in the United States for hemophilia.

Men with severe or moderately severe hemophilia B continue to display low bleeding rates and very little use of replacement therapies for at least three years following a single dose of the approved gene therapy hemgenix.
Marstacimab is being reviewed to prevent or reduce the frequency of bleeding episodes in people with hemophilia A or B. The FDA has set an action date in the first quarter of 2024.

CTTP is rare hereditary blood clotting disorder stemming from a disease-causing mutation in the ADAMTS13 gene. This gene plays a crucial role in producing the ADAMTS13 enzyme, responsible for regulating blood clotting.
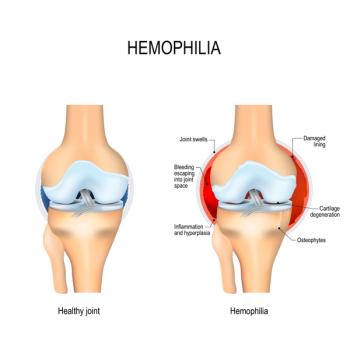
People with moderate or severe hemophilia had worse joint status, lower pain thresholds, and poorer quality of life compared with people with mild hemophilia or healthy controls.

Harsha Rajasimha, MD, founder and executive chairman of IndoUSrare, spoke to MHE about the current disparities in diversity and ethnicity in rare disease clinical trials in the U.S., as well as what he's seeing in clinical trials for diseases like hemophilia, in specific.

Expensive gene therapy has grabbed the headlines, but three promising drugs that work in the coagulation pathway are in late-stage development.

A hemophilia expert makes some comparisons between the two approved gene therapies for hemophilia.

Data from clinical trials suggest that the vast majority of the patients respond to the gene therapy for hemophilia A, says Courtney Thornburg, M.D., M.S. But there is also decreasing factor expression.

Roctavian for hemophilia A and Hemgenix for hemophilia B could spare some patients from treatment that involves intravenous infusions of clotting factors several times a week. But a hemophilia expert cautions that the high-priced gene therapies aren’t a cure and that the reprieve won’t last a lifetime.
The National Bleeding Disorders Foundation (NBDF) has joined a petition demanding that Payer Matrix stop representing itself as a patient advocacy organization.
Specialty pharmacies can help patients with hemophilia A maintain hemostasis and ensure patient safety through medication therapy management, utilizing regional care coordinators and direct pharmacist outreach, and by maintaining consistent provider communication.