
Children with serious eye conditions are at an increased risk of developing mental health conditions, including schizophrenia, anxiety, depression and bipolar.

Children with serious eye conditions are at an increased risk of developing mental health conditions, including schizophrenia, anxiety, depression and bipolar.
The CDC’s Timothy M. Uyeki, M.D., cautioned, however, that if the virus changes, and especially if it begins to infect pigs, that would be a game changer, allowing the virus to mutate to one that is more of a threat to people.

David Joyner has been appointed president and CEO of CVS Health effective immediately.

New research finds that the financial burden of treatment for age-related macular degeneration can lead to delayed or incomplete treatment and poor outcomes. Telehealth and remote monitoring could reduce gaps.

Recent outbreaks of infections related to procedures done outside the United States, such as the fungal meningitis outbreak last year related to cosmetic surgery in Mexico, demonstrate the risks of medical tourism.

The Centers for Disease Control and Prevention provides funding for HIV prevention for the populations at the highest risk of infection. State efforts to shift priorities could lead to poorer outcomes, more deaths and increased costs.

Identifying the priority populations for PrEP is crucial for guiding the future HIV prevention options, researchers said.

Interim results of a phase 2 trial indicate that the mpox vaccine MVA-VN has an immune response in adolescents similar to that of adults with no safety signals six months after the last dose.

PBMs are putting weight loss drugs, including Wegovy and Zepbound, on their national formularies, but coverage by plans is uneven. What is needed is more data about whether these drugs can lower overall healthcare costs.

Sanjula Jain of Trilliant Health talks about how employers are best positioned to demand value for money from the U.S. healthcare system.

The International AIDS Society's meeting, AIDS 2024, the 25th International AIDS Conference, was held July 22-26 in Munich, Germany. Approximately 10,000 people attended the meeting, the largest in the world devoted to HIV and AIDS. Many of the sessions were live streamed and could be viewed remotely. Here is a sample of our coverage of the meeting.

The American Society of Retina Specialists (ASRS) held its annual scientific meeting July 17-20 in Stockholm, Sweden. Here is a sample of our coverage.

UnitedHealthcare is adding deductibles to Part D prescriptions on certain formulary tiers as a result of plan design changes from the Inflation Reduction Act.

The 2024 Pharmacy Benefit Management Institute (PBMI) Annual National Conference was held Sep. 4-6 in Orlando, Florida. Managed Healthcare Executive is the official publication of the institute. Here is a sample of our coverage.
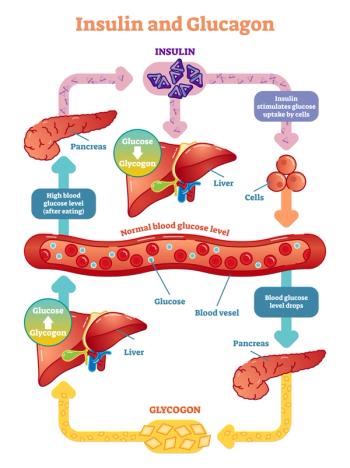
A study in mice finds that blocking somatostatin could restore the ability of the pancreas to release glucagon in the event of low blood sugar.

Blue Shield of California is purchasing Idacio for a net price of $525 per monthly dose, and most plan members will pay $0 out of pocket.
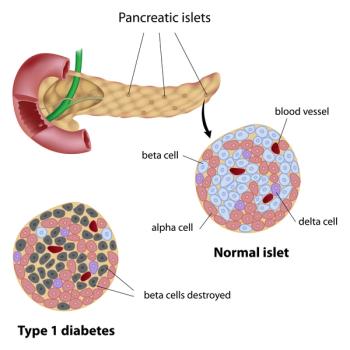
VX-880 is an allogeneic stem cell-derived islet cell therapy that has been able to control blood glucose as well as natural human islets in a small group of patients with type 1 diabetes.

PSG's Morgan Lee talks about how customers of the Big 3 PBMs report lower satisfaction across most measures, and they are less likely to recommend their PBM.

The FTC said it is focusing on insulin as the “poster child” of a broken system, where PBMs leverage formulary placement to receive higher rebates from pharmaceutical manufacturers.

GeneCQ uses integrated medical and pharmacy claims data to provide insight into the exposure to the costs for gene therapies.

A news release about the complaint says the "big 3 " pharmacy benefit managers — CVS Caremark, Optum Rx and Express Scripts — have kept hundreds of millions of dollars of rebates while excluding insulin products with lower list prices from their formularies.

When CMS begins negotiations for physician-administered drugs under the Inflation Reduction Act, providers, including those whose services are covered by commercial insurance, could see lowered reimbursement.

Whether Express Scripts wins its unique defamation lawsuit will depend on whether the PBM can prove the FTC made false statements. But winning the lawsuit may not be what the PBM is looking for.

Express Scripts alleges in its suit that the FTC followed “prejudice and politics, not evidence or sound economics,” and that there is no support for the assertion that the power of PBMs has increased over time.

The new program, which begins in January 2025, provides members of Prime Therapeutics support for navigating utilization management requirements and finding financial assistance.
An Ohio State University survey found that many adults are hesitant about getting flu, COVID-19, pneumococcal and respiratory syncytial virus (RSV) vaccines.
The FDA has identified a probable case of serious drug induced liver injury that occurred in a woman in the United States who had received Veozah. The agency is requiring additional liver blood testing after starting therapy.

Tremfya, a monoclonal antibody that blocks IL-23, is also approved to treat patients with plaque psoriasis and active psoriatic arthritis.
Pharmaceutical companies are making investments in research of therapies that target three different antigen-binding sites. Trispecific antibody research is still in its earliest phases and is focused on applications in cancer, inflammatory conditions and infectious diseases.

Insulin efsitora was able to lower A1C in patients with type 1 diabetes but more patients experienced severe hypoglycemic events than patients taking insulin degludec.