
Opdivo Qvantig is the first subcutaneously administered PD-1 inhibitor approved by the FDA. It is expected to be available in early January 2025 and will be priced at parity to the IV version of Opdivo.

Opdivo Qvantig is the first subcutaneously administered PD-1 inhibitor approved by the FDA. It is expected to be available in early January 2025 and will be priced at parity to the IV version of Opdivo.
Tryngolza is once-monthly, subcutaneous RNA-targeted therapy and is expected to be available by the end of the year with a price of $595,000.

The Institute for Clinical and Economic Review assessed the formularies of 11 payers, covering 57 million people, to determine access for drugs that the organization had reviewed in 2022 for cost-effectiveness.

Women are more than twice as likely to develop post-traumatic stress disorder than men, and a new study demonstrates for the first time that women have a greater inherited biological risk.

A new bill in Congress would require pass-through prices in both Medicare, and Medicaid and require transparency of PBM practices in the commercial space.

The 2024 Pharmacy Benefit Management Institute (PBMI) Annual National Conference was held Sep. 4-6 in Orlando, Florida. Managed Healthcare Executive is the official publication of the institute. Here is a sample of our coverage.
New research suggests that activation of the PPARδ protein, which is linked to inflammation, could treat or prevent diabetic cardiomyopathy, a serious heart condition.
In 2023, there was lower net spending and prices on adalimumab, which was likely because of rebates from AbbVie, Humira’s manufacturer, a new analysis finds.

The Institute for Clinical and Economic Review has identified five drugs — Biktarvy, Darzalex, Entresto, Cabometyx, and Xeljanz — with prices increases that are not supported by new clinical evidence, with a total of $815 million in added costs to U.S. payers in 2023.

Beginning in January 2025, Optum Rx will remove from several Humira biosimilars from its formulary, including Hyrimoz (adalimumab-adaz), the unbranded Hyrimoz and Cyltezo (adalimumab-adbm).
The biggest advantage of suzetrigine would be that it helps patients avoid the use of opioids for acute pain. The FDA’s goal date for suzetrigine is Jan. 30, 2025.

PBMs are putting weight loss drugs, including Wegovy and Zepbound, on their national formularies, but coverage by plans is uneven. What is needed is more data about whether these drugs can lower overall healthcare costs.
Patients with type 1 diabetes were more likely to be prescribed continuous glucose monitoring compared with those with type 2 diabetes, a new analysis finds.

Whether this proposed rule actually has any impact remains to be seen, especially with a new administration led by Donald Trump’s new health appointees.

CVS Caremark has removed several diabetes drugs favor of newer products and generics, and is even favoring an insulin infusion system developed by a company that was cofounded by Alan Lotvin, a former executive at CVS Health.

An intervention program that coaches patients in stress management and goal setting, along with a digital app, helped adolescents experience less distress and improved self-management behavior.

After a three-year negotiation, the FDA has dropped its objection to allowing patients to self-titrate dosing of smoked cannabis. But regulators want to see additional information about the device that will be used for inhalation.

Mehmet Oz, M.D., is another unconventional pick by Trump and is perhaps another an indication that Trump intends to let his HHS secretary nominee, Robert F. Kennedy Jr., "go wild on health."

Monjuvi is available under an accelerated approval to treat adults with relapsed or refractory diffuse large B-cell lymphoma.

Kebilidi is a one-time gene therapy administered directly to the brain in patients with AADC deficiency, rare genetic disorder.
Almost half of those surveyed by Navitus Health Solutions said they’ve been unable to fill a needed prescription because of cost.

An FDA advisory committee last year agreed that the data do not support phenylephrine as an effective nasal decongestant.

Clinicians have very strong concerns about the cost of biologics to treat food allergies, but despite these concerns, enthusiasm in the field for their use remains very high.
In the ESSENCE trial, semaglutide improved liver fibrosis in patients with metabolic dysfunction-associated steatohepatitis (MASH).

Committee members said there was uncertainty around sotagliflozin in patients with kidney disease. The FDA is currently reviewing the oral therapy as an adjunct to insulin to help control glycemic levels in adults with type 1 diabetes and chronic kidney disease. The agency’s goal date is Dec. 20, 2024.

A clinical trial in the United Kingdom has found that Stelara may help to reduce the level of a type of immune cell, which appears to play a role in destroying insulin-producing cells.
ICER has given tabelecleucel a rating of A, indicating the T cell therapy for Epstein-Barr virus related post-transplant lymphoproliferative disease has a high certainty of substantial net health benefit and would be cost-effective if priced between $143,900 and $273,700.

Qsymia’s manufacturer also released postmarketing data showing the oral therapy for weight loss was associated with reductions in 24-hour mean systolic blood pressure.

A recent study has found that biologics may have an effect on arterial wall inflammation and plaque composition, which offsets the influence of joint-derived inflammation in patients with arthritis.
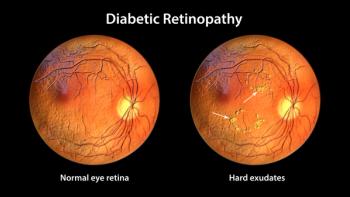
Clinical and other trials for GLP-1 therapies have found an association between these drugs and diabetic retinopathy complications in patients. But this could be an early worsening of disease unrelated to treatment.