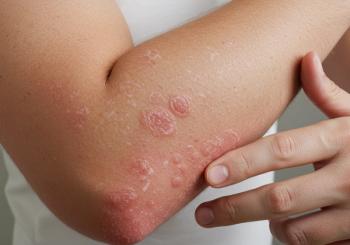
Icotrokinra is a first-in-class targeted oral peptide that blocks interleukin (IL) 23 and its receptor, which plays a key role in the inflammatory response in plaque psoriasis.

Icotrokinra is a first-in-class targeted oral peptide that blocks interleukin (IL) 23 and its receptor, which plays a key role in the inflammatory response in plaque psoriasis.

Elevidys is a gene therapy approved to treat Duchenne muscular dystrophy. Last month, Sarapta halted sales of the therapy for non-ambulatory patients.

In early research, OCU410ST, a gene therapy for Stargardt disease, demonstrated slowed lesion growth by 48% at the 12-month follow-up in evaluable treated eyes.

The combination of RPI, a genetically engineered herpes simplex virus type 1, and Opdivo shrank both injected and non-injected tumors by 30%.
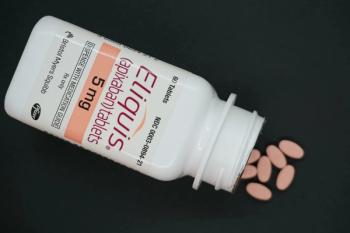
Following in the footsteps of other pharmaceutical companies, Bristol Myers Squibb and Pfizer are offering Eliquis for cash-paying patients at a discount of 40% off the list price.
A class of drugs, MDM2 inhibitors, being studied as cancer therapeutics may accidentally damage the protective barriers in the brain and eyes, finds new research in mice.

Despite the recent approval of lenacapavir as a twice yearly PrEP, there is still a need for choice in HIV prevention, argue speakers at a session at the International AIDS Society meeting.

Global health leaders and advocates challenged attendees of IAS to support the work of the partnerships that are creating patient-focused innovations for HIV prevention and treatment.

Increasing the use of Apretude among Black women requires not only expanding awareness of the long-acting PrEP regimen in various clinics but also providing more flexible scheduling and partnering with specialty pharmacies.

Barbie wears a continuous glucose monitor and an insulin pump and has a phone displaying a CGM app.
After a one-year follow-up, 10 of 12 patients given the islet cell therapy zimislecel no longer required insulin. Vertex officials said regulatory submissions are expected in 2026


Employers, some lawmakers and others have criticized pharmacy benefit managers (PBMs), especially the "big three," for having complicated, sometimes hidden practices. The PBMs say they have responded with programs and policies that add transparency to what they do.

For payers, claims for rare disease treatments used to be uncommon. Not anymore, thanks to an increasing number of new, high-priced drugs for rare diseases.

Optum Rx officials said they now have eliminated reauthorization requirements for more than 140 chronic disease medications.

A separate Prime analysis, however, of year-over-year claims data for Wegovy and Zepbound only found an improvement in adherence in 2024, suggesting there is an evolving understanding of obesity treatment.

A new study finds that enrollees who had difficulty accessing care in Medicare Advantage plans were more likely to switch to traditional Medicare than to another MA plan.

More than 50 insurers have pledged to streamline and simplify the prior authorization process through six new commitments.
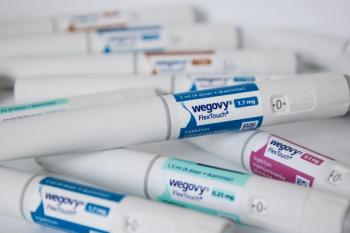
The FDA had resolved the shortage of Wegovy in April, and telehealth providers were advised to stop selling compounded semaglutide products. Novo Nordisk said that Hims & Hers continues to offer these compounded drugs.

Researchers at Yale School of Medicine have determined that a panel of biomarkers can help physicians better understand kidney disease in children.
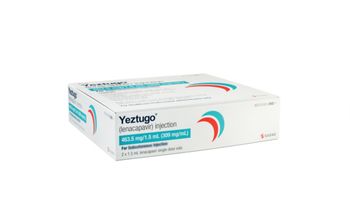
More than 99% effective at preventing HIV in trials, Yeztugo (lenacapavir) is now the first and only twice-yearly option for pre-exposure prophylaxis (PrEP).
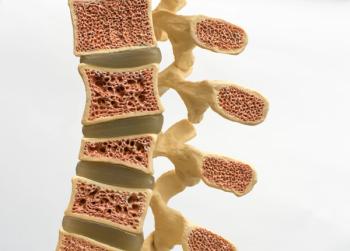
Wyost and Jubbonti are interchangeable with Xgeva/Prolia and are approved for all of the same indications in bone cancer and osteoporosis.

A second patient with Duchenne muscular dystrophy has died from acute liver failure after receiving the gene therapy Elevidys.

In an interview, James D. Chambers, Ph.D., from Tufts Medical Center, talks about the factors that are impacting the biosimilar market.

GLP-1 drugs such as Ozempic and Mounjaro outperformed metformin, statins and insulin in lowering rates of age-related macular degeneration and primary open-angle glaucoma.
A cloud-based digital twin simulation, coupled with automated insulin delivery, allows patients with diabetes to experiment with their own data on glycemic control in a safe simulation environment before adjusting their system.

Patrick Roberts, Pharm.D., said his career changed when he listened to a mentor encouraging him to go back to school.

A survey by PSG has found that the pressure to control drug costs is leading some payers to look into unbundling pharmacy benefits as a way for better control.

Switching to Humira biosimilars saved Navitus clients more than $315 million in upfront costs in 2024 and resulted in a 60% reduction in net costs per claim.

Justin Jasniewski, MBA, shared a story about his career turning point and how it involved a rejected proposal and a Big Three PBM seven-figure check.