So far, five biosimilars that reference Eylea 2 mg have been approved by the FDA. Alvotech/Teva expect to regulatory approval for its biosimilar to be completed in the fourth quarter of 2025.

So far, five biosimilars that reference Eylea 2 mg have been approved by the FDA. Alvotech/Teva expect to regulatory approval for its biosimilar to be completed in the fourth quarter of 2025.

Samsung Bioepis’ biosimilars Ospomyv and Xbryk have been approved to be interchangeable with Prolia/Xgeva treat osteoporosis and multiple myeloma/bone metastases from solid tumors.

Those voting to support Robert F. Kennedy Jr as Secretary of HHS include Louisiana Republican Sen. Bill Cassidy. Mitch McConnell, a Republican from Kentucky, however, voted no.

Just as Andrew Witty of UnitedHealth Group did in a call with investors last month, CVS Health’s CEO David Joyner defended the role of PBMs in healthcare.

CVS Health’s healthcare benefits segment, which includes Aetna, had medical benefit ratio of 92.5% in 2024, because of higher utilization, an unfavorable impact of Medicare Advantage star ratings payment and higher levels of care in Medicaid.

The PDUFA action date for an FDA decision for linvoseltamab to treat adult patients with relapsed/refractory multiple myeloma is July 10, 2025.

Emblaveo will be available in the third quarter of 2025 to treat patients with intra-abdominal infections, a common cause of sepsis in the intensive care unit.
COUR Pharmaceuticals is about begin a phase 1b/2a study to assess a therapy for type 1 diabetes that prevents islet cell destruction and triggers a T cell response.

Lindsay Bealor Greenleaf, head of Federal and State Policy at ADVI Health, talks with Managed Healthcare Executive about Robert F. Kennedy Jr., President Donald Trump’s HHS secretary nominee.
Susvimo is also approved to treat age-related macular degeneration, and the medication, ranibizumab, is delivered though a refillable ocular implant.
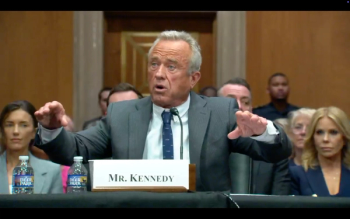
The Senate Finance Committee voted 14-13 along party lines this morning to send the nomination of Robert F. Kennedy Jr. to be HHS Secretary to the full Senate.

The new biosimilar, Celltrion’s Avtozma, was approved in both IV and subcutaneous forms to treat inflammatory conditions such as arthritis.
The HYPERION study was evaluating Winrevair in recently diagnosed adults with pulmonary arterial hypertension.
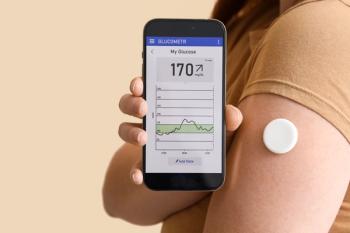
In a new study, researchers found that time-in-range for healthy blood sugar could be a viable marker of glycemic control and potentially could be used to predict complications of type 1 diabetes.

At today's confirmation hearing, Democratic senators were skeptical of the HHS Secretary nominee Robert F. Kennedy Jr.'s claim that he is now pro-vaccine.

Robert F. Kennedy Jr., the HHS secretary nominee, has said he is not antivaccine, but he has a track record of casting doubt on the safety and efficacy of vaccines.

Chronic kidney disease affects about 37 million adults in the United States and is expected to rise. It is a common complication of type 2 diabetes.

Wendy Watson takes over Kaiser Foundation Health Plan and Hospitals of the Northwest, and Corwin N. Harper becomes president of Kaiser Foundation Health Plan of Georgia.

Trump has reversed some of President Biden’s initiatives, including $2 monthly out-of-pocket cap on some generics and experimental pricing models for gene therapies. But so far the healthcare elements of the Inflation Reduction Act remain unchanged.

Health policy leaders say withdrawing from the WHO is a global health crisis in the making.

CEO Andrew Witty promised that UnitedHealthcare would work to speed up turnaround times for approval of some procedures and services in Medicare Advantage plans.

Ozempic, Rybelsus and Wegovy top the list of the newest drugs selected for Medicare Part D price negotiation, part of the Inflation Reduction Act.

In an investor call, Andrew Witty promised full transparency and 100% pass-through rebates for prescription drugs by 2028.

UnitedHealth Group reported a medical care ratio in 2024 of 85.5% compared with 83.2% in 2023.
Sana Biotechnology’s hypoimmune platform enables it to make a series of genetic modifications to cells, rendering the cells invisible to a patient’s immune system.

The Federal Trade Commission suggests that PBMs profit from specialty generic drugs such as those for cancer and HIV and steer patients to their own pharmacies.

Effective Jan. 1, 2025, Independence Blue Cross has begun excluding coverage for 18 months for therapies — but not cancer drugs — that have an accelerated approval based on a surrogate endpoint.

The updated guidelines from the American Diabetes Association emphasize the use of antibody-based screening for type 1 diabetes and recommend that physicians discuss potential use of Tzield, a monoclonal antibody approved to delay the onset of the symptoms of the disease.

Deramiocel is a cell therapy that has healing effects in muscle cells. If approved, deramiocel would be a once-quarterly therapy and the first specifically approved for cardiomyopathy in patients with Duchenne muscular dystrophy.

In November 2024, a regulatory advisory board said the benefits of Zynquista does not outweigh the risks in adults with type 1 diabetes and chronic kidney disease.