Stem Cell Transplant Shows Potential in Early Research for Type 1 Diabetes
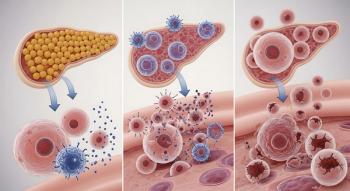
VX-880 is an allogeneic stem cell-derived islet cell therapy that has been able to control blood glucose as well as natural human islets in a small group of patients with type 1 diabetes.
In a small study, patients with type 1 diabetes who received an islet cell therapy were able to produce insulin by day 90, according to data presented at the American Diabetes Association 84th Scientific Sessions held in June 2024.
“Stem cell-derived islets regulate blood glucose control as well as natural human islets,” said Piotr Witkowski, M.D., Ph.D., professor of Surgery and Director, Pancreatic, and Islet Transplant Program, University of Chicago Medicine, one of the investigators on the study, said in a
Type 1 diabetes is an autoimmune disease that affects about 2 million Americans, about 5% to 10% of all diabetes cases, according to the Centers for Disease Control and Prevention (CDC). In type 1 diabetes, the pancreas does not make insulin. A
Developed by Vertex Pharmaceuticals, VX-880 is an investigational allogeneic stem cell-derived islet cell therapy. VX-880 is delivered by an infusion into the liver portal vein. The therapy aims to replace the insulin-producing cells that have been destroyed in people with type 1 diabetes. The treatment, however, requires chronic immunosuppressive therapy to protect the islet cells from rejection.
The phase 1/2 trial is an open-label study patients who have type 1 diabetes with severe hypoglycemia an impaired hypoglycemic awareness, a limited ability to recognize the onset of hypoglycemia. In the trial, 12 patients were enrolled and received the full dose of VX-880 as a single infusion in Parts B and C of the trial.
Researchers found that all 12 patients demonstrated islet cell engraftment, and at the latest visit all patients had improved glycemic control and achieved ADA-recommended targets for both HbA1c below 7.0% and time-in-range above 70% on continuous glucose monitoring.
Nearly all participants (11 of 12) had a reduction or elimination of exogenous insulin use at their last visit. All patients had elimination of severe hypoglycemic events during the evaluation period.
Three patients had at least one year of follow-up and were able to be evaluated for the primary endpoint. These three patients met the primary endpoint of elimination of severe hypoglycemic events (from day 90 after infusion) with HbA1c <7.0% and the secondary endpoint of insulin independence.
The majority of adverse events were mild or moderate, and there were no serious adverse events related to VX-880 treatment. The safety profile is generally consistent with the immunosuppressive regimen used in the study, the infusion procedure, and complications from long-standing diabetes.
Vertex officials noted in the news release that the trial is being expanded to enroll about 37 patients.
Vertex is also developing a cell therapy to treat adults with type 1 diabetes based on a different approach. VX-264 encapsulates these same islet cells in a device to be surgically implanted in the body. These devices are designed to shield the cells from the body's immune system. A phase 1/2 trial is ongoing, and Vertex has completed Part A of this study. Part B of the phase 1/2 is under way and enrolling and dosing. In Part B, patients receive the full target dose with a stagger period between patients, and in Part C, patients will receive the full target dose with no stagger.
Newsletter
Get the latest industry news, event updates, and more from Managed healthcare Executive.