
UnitedHealth has found 22 screenshots, allegedly from Change Healthcare files, that were posted for about a week on the dark web. Some of these contained personal health information. The extent of data release is not yet known.

UnitedHealth has found 22 screenshots, allegedly from Change Healthcare files, that were posted for about a week on the dark web. Some of these contained personal health information. The extent of data release is not yet known.
ImmunityBio’s Anktiva is an antibody cytokine fusion protein approved to treat patients with non-muscle invasive bladder cancer.
Entyvio as an injection and subcutaneous formulations is approved to treat both Crohn’s and ulcerative colitis.

Spending on the GLP-1 class of therapeutics is expected to grow by 378% to $8.1 billion by 2027.

Up to 50 generics could be approved this year, including Victoza to treat patients with diabetes. If generics of Victoza become available, this would the first GLP-1 to face generic competition.

Innovative contracts are becoming more common for gene therapies. They aim to balance a therapy’s cost-effectiveness and affordability, as well as address the uncertainty of long-term benefit.

Panelists discussed reaching beyond large patient advocacy groups for input and how clinical trial endpoints may paint an incomplete picture of a drug's true value to patients.
The pipeline for NASH treatments is robust, with six therapies in phase 3 development. Speakers at the annual AMCP meeting discuss Rezdiffra, the first drug approved specifically for NASH, and how to think about coverage issues.

Use of GLP-1 drugs and weight-loss care management programs need to focus on adherence and persistency for better health outcomes, suggests Prime Therapeutics/MagellanRx Management poster at AMCP 2024.

In a slow week, the FDA has approved Fasenra for children 6 to 11 years of age with eosinophilic asthma, a rare form of asthma.

Jeffrey Casberg, M.S., R.Ph., a senior vice president of clinical pharmacy at IPD Analytics LLC, a drug intelligence firm that advises payers and pharmaceutical companies, talks about how payers are thinking about weight-loss drugs.
The FDA is reviewing two novel therapies: a psychedelic-assisted therapy for PTSD with a target action date of Aug. 11, 2024, and therapy for schizophrenia that does not directly block dopamine receptors with an action date of Sept. 26, 2024.

Ventegra, which provides drug benefit solutions, is also converting to a nonprofit status, which will be completed June 30, 2024.
The FDA is currently reviewing tarlatamab for small-cell lung cancer with a review date in June and datopotamab deruxtecan for non-small cell lung cancer with a review date in the fourth quarter.

New study finds trial enrollment was highest at NCI-designated cancer centers. Researchers said more needs to be done to enroll patients from community cancer sites.

A new study finds that of the patient portal messages that result in a bill, most involved physicians helping patients with high blood pressure or diabetes.

Caps on Medicare Part D cost sharing as a result of the Inflation Reduction Act, could reduce members’ financial incentive for switching to a biosimilar, suggests the newest Samsung Bioepis Quarterly Biosimilar Market Report.

The FDA has approved several new therapies this week, including Voydeya as an add-on therapy for blood disorder PNH; Zevtera for serious bacterial infections; Fanapt for bipolar 1; and the CAR-T therapy Abecma for earlier treatment in multiple myeloma. The agency has also accepted a supplemental BLA for Bimzelx in hidradenitis suppurativa and set a review date for datopotamab deruxtecan in breast cancer.

The quality-adjusted life-years tool has been misunderstood, Dr. Joshua Cohen says. QALYs are a measure of the value that comes from restoring a person to health, not about discrimination.

Between 2011 and 2020, 45.6% of new cancer drugs were approved through the accelerated approval pathway, according to a new report from the American Association of Cancer Research.

The FDA is also considering a separate BLA for a 2 mL syringe. Bimzelx is already available to treat plaque psoriasis as a 1 ml syringe; a dose is two subcutaneous injections with a cost of $7,200 per syringe.


In 2025, the out-of-pocket cap and other Part D related provisions in the Inflation Reduction Act are projected to save women in Medicare an average of 28% in out-of-pocket costs, according to a new analysis from the Office of the Assistant Secretary for Planning and Evaluation.

In 2025, the out-of-pocket cap and other Part D related provisions in the Inflation Reduction Act are projected to save women in Medicare an average of 28% in out-of-pocket costs, according to a new analysis from the Office of the Assistant Secretary for Planning and Evaluation.

Researchers found that pancreas volume, coupled with metabolic measures, can better predict disease progression of type 1 diabetes than either MRI imaging or glucose testing alone.

Foluso Agboola discusses the framework that the Institute for Clinical and Economic Review has developed for assessing whether clinical trial populations represent the real-world patient population.
Vafseo, an oral therapy to treat patients with anemia due to chronic kidney, will be available in January 2025. About 40% of patients who could be treated with Vafseo have coverage through Medicare Advantage plans.

Ozempic and Rybelsus could become a target for Medicare price negotiation because of high spending and additional approved uses of the GLP-1 therapies.
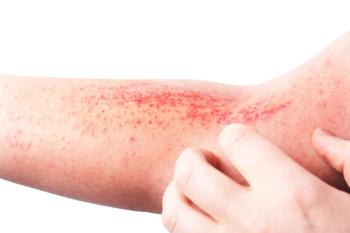
Adults and children with atopic dermatitis experience worse anxiety and depression as disease becomes more severe.
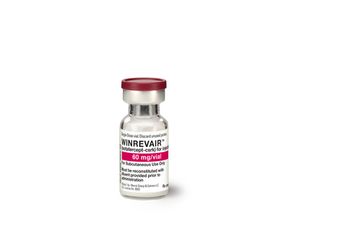
Sotatercept — now with the brand name Winrevair — will be available through specialty pharmacies by the end of April. The price of Winrevair is $14,000 per vial.