
Terlivaz is the first approved therapy to treat hepatorenal syndrome, a rare form of kidney disfunction related to advanced liver disease.

Terlivaz is the first approved therapy to treat hepatorenal syndrome, a rare form of kidney disfunction related to advanced liver disease.

The additions include apps to help people better manage their sleep issues, anxiety, alcohol and opioid use disorders, and inflammatory conditions.
Aripiprazole, currently available as a once-monthly injection under the brand name Abilify Maintena, is being reviewed as a two-month therapy. The FDA target date for completion of the review is April 27, 2023.

The FDA has granted priority review of velmanase alfa, an enzyme replacement, to treat patients with alpha-mannosidosis, a rare genetic disease. The FDA has assigned an action date in the first half of 2023.

Jeffrey A. Shaman, Ph.D., chief science officer at Coriell Life Sciences, talked about how pharmacogenomics can inform medication management.
If approved, Perrigo’s Opill could be the first-ever over-the-counter birth control pill. The advisory committee meeting is scheduled for Nov. 18, 2022.

If approved, trofinetide will be the first drug available for the treatment of Rett syndrome, a rare, genetic neurological disorder mostly in girls. The FDA action date is March 12, 2023.

Kali Panagos, Pharm.D., at ARMSRx, discusses the growing trend of copay assistance programs and alternative funding solutions that can help provide access to expensive specialty therapies.
A Texas judge ruled that coverage for HIV pre-exposure prophylaxis (PrEP) violates religious freedom but there is concern that cancer screenings and other preventive services could be affected.

Developed by Bristol Myers Squibb, Sotyktu is the first approved TYK2 inhibitor. It is expected to be available later this month.

Rolvedon, which previously had the brand name Rolontis, is the first novel long-acting GCSF product approved in more than 20 years. Spectrum Pharmaceuticals expects it to be available in the fourth quarter.

The FDA is expected to make a decision on Pfizer’s ritlecitinib in the second quarter of 2023.

Because of a generic is now available, Toviaz has been removed and up-tiered on Prime’s Medicare formularies.

Daxxify enters the injectable botulinum toxin market as a twice-a year treatment for frown lines.
Although Xeljanz, Olumiant and Rinvoq are in the same drug class, the risk of serious adverse events from these three products to treat rheumatoid arthritis and other inflammatory conditions may not be similar.

Dr. Reddy’s received a first-to-market 180 days of exclusivity for the 2.5 mg and 20 mg strengths of its generic lenalidomide capsules.

In a second committee meeting, FDA advisors supported approval of AMX0035 after the company presented additional analysis of phase 2 data of AMX0035 to treat patients with ALS. The Prescription Drug User Fee Act (PDUFA) target action date is Sept. 29, 2022.
Prime Therapeutics’ predictive model was able to identify members with high breast cancer pharmacy and medical claims to help clients better manage drug spend.
If approved, SER-109 to treat recurrent C. diff infections could be the first-ever FDA approved oral microbiome therapeutic.

Fresenius Kabi expects to launch Stimufend as a prefilled syringe early in 2023.

The FDA would not issue an emergency use authorization for Eiger BioPharmaceuticals’ peginterferon lambda to treat patients with mild-to-moderate COVID-19.
If approved, NOV03 would be the first prescription eye drop to address excessive tear evaporation. The FDA has assigned a PDUFA action date of June 28, 2023.
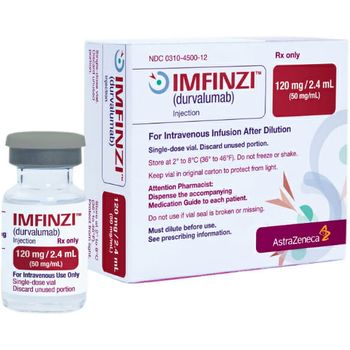
A phase 3 trial showed that Imfinzi in combination with chemotherapy reduced the risk of death by 20% compared with chemotherapy alone.

Konvomep, an oral liquid of omeprazole and sodium bicarbonate, will be available in the first quarter of 2023

With this approval, about 300 children between 12 months and 24 months will be eligible for treatment with Orkambi.
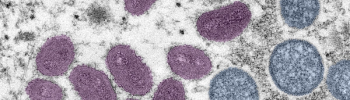
HHS is providing $11 million to Grand River Aseptic Manufacturing to accelerate manufacturing of the Jynneos monkeypox vaccine. The facility will be operational later this year.