
OptumRx will offer Amjevita and two other biosimilars alongside Humira. OptumRx is the first PBM to make public its plans for the seven Humira biosimilars that could be introduced in 2023.

OptumRx will offer Amjevita and two other biosimilars alongside Humira. OptumRx is the first PBM to make public its plans for the seven Humira biosimilars that could be introduced in 2023.
Launched in August, Zoryve is a once-daily, steroid-free cream and is the first topical PDE4 inhibitor approved to treat plaque psoriasis in patients 12 years of age or older.
If approved, Xphozah will be the first phosphate absorption inhibitor for adults with chronic kidney disease who are on dialysis. An FDA decision is expected within 30 days.

Sun Pharma will market SPARC’s phenobarbital, which is under FDA review to treat neonatal seizures. In addition, several new generics have been approved, including a generic of Novartis’ Gilenya for multiple sclerosis and AbbVie’s Lupron for prostate cancer.

The agency is encouraging sponsors to submit applications for low-dose, nonprescription naloxone products.

After review of phase 3 data, GSK will limit the use of Zejula in the second line to those with inherited BRCA mutations.

OptumRx now requires prior authorization for 16 products, including 11 that are used to treat patients with diabetes.

Elahere is a first-in-class antibody-drug conjugate targeted against folate receptor alpha, a protein on the surface of ovarian cancer cells.

This is second issue related to Omnipod. Last week, the company announced that it had received 50 complaints related to the batteries of Omnipod DASH Personal Diabetes Manager.

The approval extends the use of Liletta for an additional two years. It is the only hormonal IUD approved for continuous use up to eight years.

Zilucoplan is a targeted therapy that inhibits key components in the underlying disease pathology. The anticipated PDUFA date is in the fourth quarter of 2023.

PRX-102 is an enzyme replacement therapy for Fabry disease, a rare genetic disorder.

Imjudo is a monoclonal antibody that targets the activity CTLA-4 that was approved to be used in combination with Imfinzi, a PD-L1 inhibitor, for patients with non-small cell lung cancer.
IPX203 is an oral formulation of carbidopa/levodopa extended-release capsules that, if approved, could provide patients with better symptom control. The FDA assigned a Prescription Drug User Fee Act date of June 30, 2023.

In a trial of patients two years and older, Adcetris plus standard of care chemotherapy led to a 59% reduction in risk of disease progression or relapse, second malignancy or death

A 10-day implementation has given the team at Abarca Health the opportunity to challenge how they manage implementations in general.
Vizient saw a 43% surge in demand for all amoxicillin products in the acute care setting from September to October, but fill rates have dropped 25%.
If approved, Aphexdra would be used along with G-CSF treatment for autologous transplantation in patients with multiple myeloma. The Prescription Drug User Fee Act target action date is Sept. 9, 2023.

Kineret has been granted an EUA for hospitalized adults with pneumonia who require supplemental oxygen.

Snezana Mahon, Pharm.D., Transcarent’s chief operating officer, discusses the company’s efforts to provide a seamless and interconnected pharmacy ecosystem.
The new indication expands the patient population eligible for a Libtayo-based regimen to include combination treatment with chemotherapy irrespective of PD-L1 expression levels.
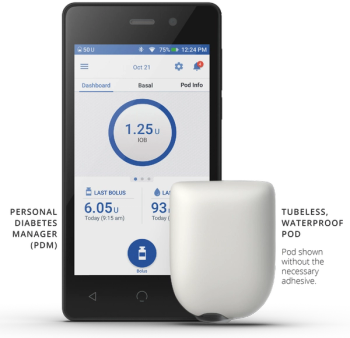
Omnipod DASH PDMs are at increased risk of malfunction if overcharged beyond the maximum battery voltage.

The regulatory agency has added the risk of rare skin infections to the warnings and precautions section of the labeling for amoxicillin products.

Bebtelovimab is used to treat COVID-19, but omicron subvariants BQ.1 and BQ.1.1 may be resistant, the FDA said.

Subscribers will be able to compare medications through Amazon and pay either their insurance copay price or the price from Prime Therapeutics’ MedsYourWay discount card.
The greatest reductions were seen in girls who were vaccinated when they were adolescents, with up to 73% reduction in cervical pre-cancerous lesions.