This is the fourth indication for Vraylar, a dopamine and serotonin partial agonist that can now be used as an adjunctive therapy in patients with major depressive disorder.

This is the fourth indication for Vraylar, a dopamine and serotonin partial agonist that can now be used as an adjunctive therapy in patients with major depressive disorder.

Cytalux, approved in November 2021 for identifying ovarian cancer lesions, is now approved to spot lung cancer during surgery.

Smaller and midsize pharmacy benefit managers are taking on Optum Rx, CVS Caremark and Express Scripts. They say they have better technology and are more transparent about their pricing.
If approved, Linzess would be the first prescription therapy for functional constipation in children and adolescents 6 to 17 years of age.
Adstiladrin offers an alternative to bladder removal surgery for patients who are at high risk of recurrence and metastatic cancer.

A decision is anticipated in early January about which of the now eight Humira biosimilars will be included.
Prescryptive will offer a subscription-based program for employers that aims to provide a more predictable benefit for companies and their employees.

Iyuzeh is a preservative-free formulation of latanoprost and doesn’t need to be stored at refrigerated temperatures. It will be available in the second half of 2023.

HyBryte is a photodynamic therapy. The active ingredient, synthetic hypericin, is topically applied to skin lesions that is taken up by the malignant T-cells and activated by visible light.

This is the eighth biosimilar to reference the blockbuster rheumatoid arthritis drug Humira that will launch in 2023.

The agency has extended the review time by three months for Lynparza in combination with abiraterone and prednisone or prednisolone.

The Medicare Part D Senior Savings Model — a CMS effort to test $35 copays for insulin — led to lowered total costs for beneficiaries.
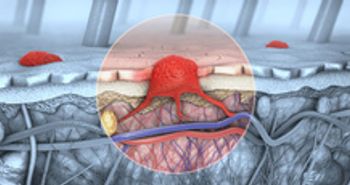
Moderna is developing an individualized cancer vaccine that uses mRNA technology to specifically target that person’s cancer.

Magellan Rx predicts oncology and high-cost rare disease treatments will continue to drive medical pharmacy spend in commercial, Medicaid and Medicare.
Building on the success of the companies’ COVID-19 vaccines, Pfizer and BioNTech have begun a phase 1 trial of a combined flu-COVID-19 vaccine.

The FDA issued an accelerated approval for Mirati’s Krazati for patients with KRAS-mutated non-small cell lung cancer. The agency also approved a companion diagnostic to identify eligible patients.

The acquisition provides Biocon Biologics with eight commercial biosimilars, and is part of Viatris’ efforts to pay down debt related to phase 1 research and the company’s acquisition of two ophthalmology businesses.
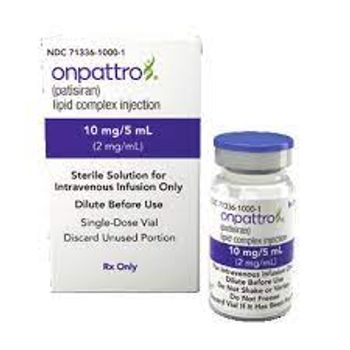
A phase 3 study demonstrated that Onpattro improved functional capacity, health status and quality of life compared with placebo in this patient population.

The acceptance of Biogen’s application follows regulator’s acceptance of an application by Fresenius Kabi for another Actemra biosimilar.
The bivalent vaccines add the omicron variant BA.4 and BA.5 to the original SARS-CoV-2 and a component of omicron lineage BA.1.
Talquetamab is a bispecific antibody that targets both CD3 on T cells and GPRC5D, which is overexpressed on myeloma cells.

Low-income subsidies can eliminate much of the out-of-pocket costs that patients must pay.

The EmsanaRx Plus program is connecting employers to the discounted drugs offered through Mark Cuban Cost Plus Drug Company. The program will be available beginning March 1, 2023
Oncopeptides disagrees with the FDA about Pepaxto and will make a decision about next steps by the end of the first quarter of 2023.

Cost savings were not associated with an increase in adverse drug events.

Independent pharmacies are no longer part of Tricare’s pharmacy network for uniformed service members and their families. The pharmacy groups say this will impact 400,000 beneficiaries.