In a real-world example, investigators found that lowered pricing of prescription drugs results in lower patient out-of-pocket costs, particularly for those patients with coinsurance.

In a real-world example, investigators found that lowered pricing of prescription drugs results in lower patient out-of-pocket costs, particularly for those patients with coinsurance.

In 2023, CVS executives expect revenue in pharmacy services to grow by 1% to 2% driven by generic and biosimilar launches.

In 2022, 12 states have enacted 19 pieces of legislation that put a variety of restrictions and requirements on pharmacy benefit managers (PBMs).

If approved, reproxalap would be the first inhibitor of RASP to treat patients with dry eye disease. The FDA PDUFA date is Nov. 23, 2023.
Takhzyro is the first therapy to prevent attacks of hereditary angioedema (HAE) in children ages 2 to under 6.

Zuranolone is a rapid-acting neuroactive steroid that can take effect in 14 days to treat patients with major depressive disorder and postpartum depression. The FDA has assigned a PDUFA action date of Aug. 5, 2023.
The products may be ineffective, unsafe and could prevent a person from seeking an appropriate diagnosis.
Trodelvy has been recommended as a preferred treatment for metastatic HR+/HER2- breast cancer by the National Comprehensive Cancer Network.

Tezspire is a biologic that specifically targets the inflammation associated with asthma.

Evernorth, Cigna’s pharmacy and benefits solutions division, contributed more than 75% of the company’s revenue last year.

The real impact may not likely be seen until after July when additional biosimilars referencing Humira are launched.

Daprodustat, now with the brand name of Jesduvroq, is a new oral therapy but it carries a boxed warning about the risk of cardiovascular events.
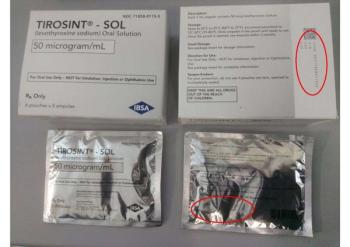
IBSA Pharma is recalling the hypothyroid medication because analyses showed lowered levels of levothyroxine in some lots.

Dosing has been lowered to reduce the risk of liver damage in patients taking Turalio, which is approved to treat patients with a rare tumor that affects the joints.

Vizient is forecasting a 3.78% rise in pharmacy prices in hospitals and non-acute care facilities, reflecting increases in both prices and utilization.

Some PBMs will offer Amgen’s Amjevita biosimilar as a preferred product alongside Humira, but CVS Caremark said Humira will remain preferred and Amjevita will be placed on a non-preferred brand tier.

The collaboration between Cost Plus Drugs and OncoPower provides access to generic medications for patients with cancer integrated with OncoPower’s Pill Reminder tool.

The proposed legislation, sponsored by Sens. Chuck Grassley and Maria Cantwell, aims to increase transparency of PBM business practices.
Elacestrant, now with the brand name Orserdu, is an oral selective estrogen receptor degrader (SERD), which works by blocking the effects of estrogen on hormone receptor-positive breast cancer cells.

Jaypirca is the first BTK inhibitor specifically approved for patients with mantle cell lymphoma previously treated with a covalent BTK inhibitor. It has a wholesale acquisition cost of $21,000 per 30 days of therapy.

This approval marks the fifth indication for Keytruda-based regimens in non-small cell lung cancer and the 34th indication in the United States.

Programs that do not count pharmaceutical company coupons and copay assistance toward deductibles have grown over the last five years, but states are stepping in to limit these programs.

AstraZeneca is developing a next-generation long-acting antibody for pre-exposure prophylaxis of COVID-19, which it plans to make available in the second half of 2023.

Evio is launching a new pharmacy solution that leverages Flipt’s proprietary, cloud-based benefit administration and patient engagement platforms.

Three out of four health plans scored a “C” or an “F” because of utilization management restrictions they place on medication for autoimmune conditions.

In this video, Dea Belazi, president and chief executive officer of AscellaHealth, discusses how payers will approach specialty drugs.