
Coherus’ Cimerli has been approved to be interchangeable for all five indications, including age-related macular degeneration and diabetic retinopathy. It will be available in early October 2022.

Coherus’ Cimerli has been approved to be interchangeable for all five indications, including age-related macular degeneration and diabetic retinopathy. It will be available in early October 2022.

Investigators found that socioeconomic and financial barriers can affect patients’ access to oral cancer medications. They suggest that addressing these barriers could improve adherence.

Prime has removed six drugs from its formularies because generics are now available. One has been removed because it has been discontinued.
Stelara is the first biologic targeting both cytokines IL-12 and IL-23 and offers a therapeutic option for children six years of age and older living with active psoriatic arthritis, a rare disease.
Optum has teamed up with Sanofi to provide savings cards for six insulins through the Optum Store.
Zoryve, the first topical PDE4 inhibitor approved to treat patients with plaque psoriasis, will be available by mid-August.

The prescription drug launches include a therapy to treat adults with multiple myeloma and a treatment for overactive bladder. Dr. Reddy’s has also acquired several therapies from Eton Pharmaceuticals, including hypotension treatments.
The trial of the immunotherapy combination, although approved in other renal cell carcinoma indications, did not meet the endpoint of disease-free survival in patients with localized disease.
If approved, N-803 — with the brand name Anktiva — plus the Bacillus Calmette-Guérin vaccine would be the first immunotherapy combination for this indication in 23 years. The Prescription Drug User Fee Act target action date is May 23, 2023.

Orsini Specialty Pharmacy has been chosen as the exclusive pharmacy provider of Ztalmy.
The bipartisan bill introduced in the Senate in May would require PBMs to report to the FTC how much money they make through spread pricing and pharmacy fees.

Benlysta is now the only biologic approved for adults and children who have lupus or lupus nephritis, an inflammation of the kidneys.

Kelly Stanton, director of quality at Qualio, talks about the impact on patients of prescription drug recalls.

This follows a move by Bayer to import Ultravist with non-U.S. labeling also to address pandemic-related shortages of iodine-containing contrast media used in imaging procedures.
AbbVie is seeking approval in the United States and Europe for Rinvoq to treat patients with Crohn’s disease.
If approved, Sandoz’ natalizumab would be the first biosimilar of Tysabri to treat patients with multiple sclerosis.
Next year, seven biosimilars of Humira are expected to launch, but leaders from the Biosimilars Forum are concerned not all will make it onto formularies.

If approved, tofersen will be the first treatment that targets a genetic cause of ALS. The FDA assigned a Prescription Drug User Fee Act action date of Jan. 25, 2023, but said it will hold an advisory committee meeting for this application.
If approved, Enhertu would offer a treatment option for patients with breast cancer who have a lower expression of HER2. The PDUFA action date is during the fourth quarter of the 2022.

Many products added to the Express Scripts preferred drug list include prior authorization and other utilization management tools.

David Lassen, Pharm.D., chief clinical officer at Prime, discusses a pilot program that used a predictive model to identify patients at high risk of overusing opioids.

The organization claims that direct and indirect remuneration fees and other clawback programs amount to a breach of contract.

Sandoz is seeking approval for a high-concentration formulation of Hyrimoz, which references AbbVie’s Humira.
The KEYNOTE-412 study did not meet its primary endpoint of event-free survival.
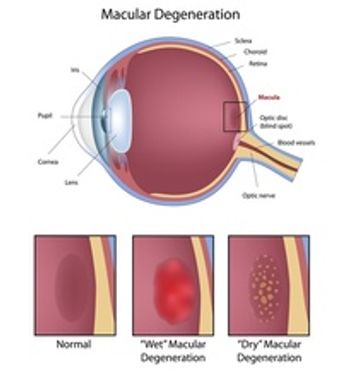
Pegcetacoplan is a targeted therapy to treat patients with age-related macular degeneration. The Prescription Drug User Fee Act (PDUFA) target action date is Nov. 26, 2022.

An independent data monitoring committee made the recommendation to stop both arms of the trial after a planned interim analysis found the trial didn’t meet its objectives.