BMS warns about the risk of extramedullary hematopoiesis, a rare complication in which the production of blood cells occurs outside of the bone marrow.

BMS warns about the risk of extramedullary hematopoiesis, a rare complication in which the production of blood cells occurs outside of the bone marrow.

Zonisade is the first oral liquid form of zonisamide to be approved by the FDA to treat patients with epilepsy.

Pfizer’s Xalkori is now approved for three indications, with the most recent being ALK-positive myofibroblastic tumors

Some insulins, as well as some drugs used in emergency care, will now be offered at $0 copay for eligible patients.

A novel therapy in early development aims to permanently turn off the PCSK9 gene in the liver and lower cholesterol with a one-time treatment. It is being developed for a genetic form of high cholesterol.
Particulates were seen in a single vial, which could lead to blockage of blood vessels, including decreased blood flow to the brain, heart attack, and pulmonary embolus.

Investigators suggest that policies that improve price transparency and increase competition for generic drugs could prevent patients and Medicare from overpaying on generic drugs.
The FDA has not been able to complete inspections of facilities because of COVID-19 restrictions.
The U.S. government pre-purchases 3.2 million initial doses of the Novavax COVID-19 vaccine.

Drugs approved with an FDA breakthrough designation can provide value that offsets their higher costs, finds study conducted by Tufts Center for the Evaluation of Value and Risk in Health.

The FDA has assigned a Prescription Drug User Fee Act action date of May 12, 2023, for [vic-]trastuzumab duocarmazine (SYD985).

In 2023, a wave of biosimilar launches is expected, including seven biosimilars of Humira.

In the future, payers plan to leverage reinsurance and value- or outcomes-based contracting to provide access to high-cost therapies.
Piclidenoson is a first-in-class, A3 adenosine receptor agonist small molecule that inhibits the inflammatory cytokines interleukin 17 and 23.
Blue Cross Blue Shield of North Carolina found that uptake of Trikafta was rapid after approval and total cost of care increased 52% despite reductions in hospitalizations and nonpharmacy costs.
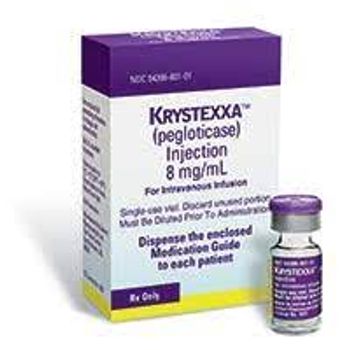
The FDA approved a supplemental BLA for use of Krystexxa with methotrexate for the treatment of uncontrolled gout.

This is the second batch to be recalled because of the potential of missing labels.
NOV03 (perfluorohexyloctane) is a first-in-class eye drop with a novel mechanism of action. If approved it will be the first to address signs and symptoms of dry eye disease.
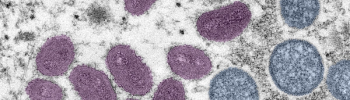
Under the national vaccine strategy, the U.S. Department of Health and Human Services is expanding access to the monkeypox vaccine, Jynneos, in areas with the highest transmission and need.

Under a new add-on program, plan members will have access to an expanded list of FDA-approved specialty generic medications with no copays.
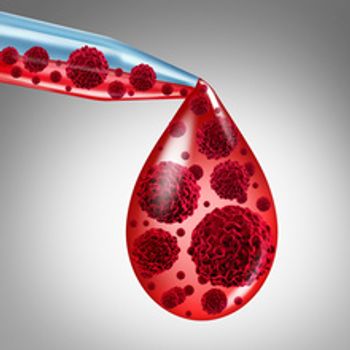
If approved, mosunetuzumab could be the first T-cell engaging bispecific antibody for the treatment of any type of non-Hodgkin’s lymphoma. The FDA has assigned a PDUFA date of Dec. 29, 2022.
Lecanemab is monoclonal antibody that targets beta amyloid to treat mild cognitive impairment. The FDA has assigned a Prescription Drug User Fee Act (PDUFA) action date of Jan. 6, 2023.
The Peripheral and Central Nervous System Drugs Advisory Committee will meet for a second time in September 2022 to discuss new analysis of the data for AMX0035 for the treatment of patients with amyotrophic lateral sclerosis.

The United States has acquired adult and pediatric doses of the COVID-19 vaccine for delivery in early fall in a contract worth $3.2 billion, as well as an additional 150,000 doses of bebtelovimab for about $275 million.

Qsymia is only available through a Risk Evaluation and Mitigation Strategy (REMS) program because of the increased risk for birth defects.