
Labor shortages and Drug Enforcement Agency quotas have led to supply shortages of Adderall and its generics, which are used to treat attention deficit hyperactivity disorder.

Labor shortages and Drug Enforcement Agency quotas have led to supply shortages of Adderall and its generics, which are used to treat attention deficit hyperactivity disorder.
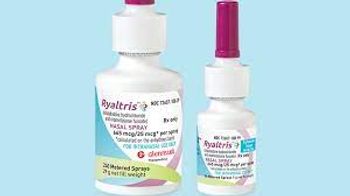
Ryaltris is a fixed-dose combination therapy that provides relief for both nasal and ocular symptoms of seasonal allergic rhinitis in a nasal spray.

The approval of the updated bivalent boosters is based on data from clinical studies of the omicron variant BA.1, and animal data on the omicron BA.4 and BA.5 variant.
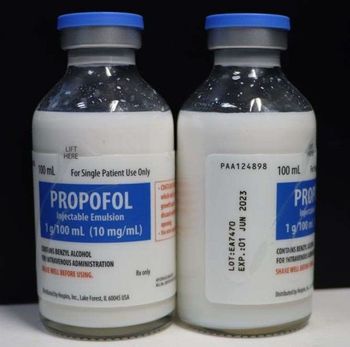
This recall of propofol because of particulates seen in vials follows a previous one in July 2022.

The direct-to-consumer pharmacy also added 13 new products, and now carries almost 1,000 medications.

The target action date for the agency’s decision for efanesoctocog alfa, a factor VIII therapy, is Feb. 28, 2023.

Survey participants said their drug costs have gone up and almost a third had trouble paying for food and housing because of high drug costs.

Hyftor was approved in April 2022 to treat patients with the rare disease facial angiofibroma associated with tuberous sclerosis complex.

Payer coverage and the prior authorization process continue to be barriers for the adoption of biosimilars, according to a new Vizient survey.

Pemazyre is the first approved treatment specifically for patients with FGFR1 rearrangement, a rare blood cancer.
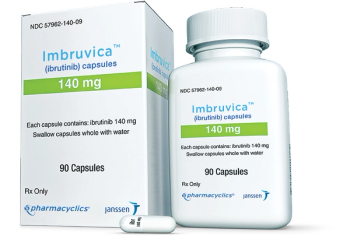
Imbruvica is the first approved treatment option for children under 12 with chronic graft versus host disease and the first BTKi therapy approved for pediatric patients.

The PBM is removing four products and adding one product back to its standard control formulary.

Pending authorization, Moderna will be ready to ship the updated bivalent booster in September 2022.
Out-of-pocket costs decreased for those with insurance but rose for those without insurance, finds RAND study.

The FDA has assigned a Prescription Drug User Fee Act action date of June 16, 2023.

Omnipod 5 is now available through retail pharmacies, as well as specialty and mail-order pharmacies.

The updated COVID-19 vaccine booster specifically addresses the omicron BA.4/BA.5 variants. If authorized, it will be available to ship immediately.

Novavax’s vaccine is the first protein-based COVID-19 vaccine authorized in the United States.
Axsome anticipates Auvelity to be commercially available in the fourth quarter of 2022.

Recent approvals include a first generic for the infertility drug Cetrotide, as well as generics of Revatio, Velcade and Esbriet.

Mirena, a long-acting hormonal IUD, was previously approved to prevent pregnancy for up to seven years.

The rate reflects a continued trend toward moderation of the overall increase in drug prices.

If approved, fezolinetant would treat patients with moderate-to-severe hot flashes and/or night sweats associated with menopause. The PDUFA date is Feb 22, 2023.

After a failed trial in advanced breast cancer, Sanofi has stopped all studies of amcenestrant, including in early-stage breast cancer.

Hadlima, a biosimilar of Humira, will be launched next year to treat patients with certain chronic autoimmune diseases.

Bluebird bio has set a wholesale acquisition cost of $2.8 million for the gene therapy beti-cel, now with the brand name Zynteglo, and is offering an outcomes-based contract with an 80% risk sharing.