
After a three-year negotiation, the FDA has dropped its objection to allowing patients to self-titrate dosing of smoked cannabis. But regulators want to see additional information about the device that will be used for inhalation.

After a three-year negotiation, the FDA has dropped its objection to allowing patients to self-titrate dosing of smoked cannabis. But regulators want to see additional information about the device that will be used for inhalation.
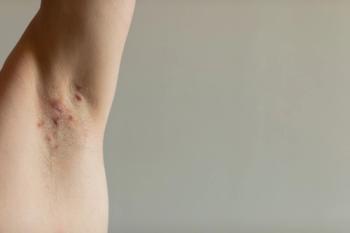
The FDA has approved UCB's Bimzelx for moderate-to-severe hidradenitis suppurativa, offering a new treatment option for this painful autoimmune skin disease.
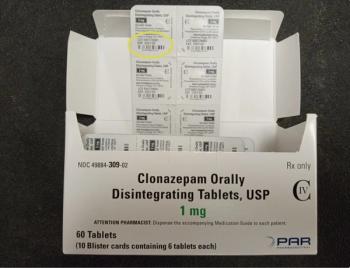
A total of 17 lots are now part of the recall of Clonazepam because some cartons have the wrong strength on the label.
Relapsed or refractory acute leukemia with a KMT2A translocation currently has an overall survival rate of less than one year when treated with frontline therapies.
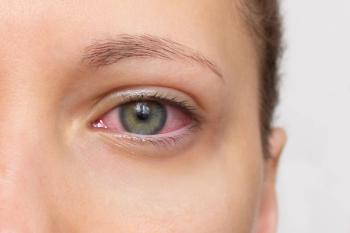
The FDA assigned a Prescription Drug User Fee Act (PDUFA) date of April 2, 2025, for reproxalap for patients with dry eye disease. Reproxalap also is being tested in allergic conjunctivitis.

Monjuvi is available under an accelerated approval to treat adults with relapsed or refractory diffuse large B-cell lymphoma.
The FDA has provided a date of April 18, 2025, to review the application for Dupixent to treat patients with chronic spontaneous urticaria, an inflammatory skin disease.
After feedback from the FDA, the companies have voluntarily withdrawn the previous biologics licensing application for datopotamab deruxtecan for patients with advanced or metastatic nonsquamous non-small cell lung cancer.
Almost half of those surveyed by Navitus Health Solutions said they’ve been unable to fill a needed prescription because of cost.
Aucatzyl is a CAR T-cell therapy that targets CD19 and has been designed to minimize excessive activation of the programmed T cells. The wholesale acquisition cost is $525,000
For the first time, Skyrizi has replaced Humira as AbbVie’s sales driver, largely due to companies encouraging “product hopping” to avoid competition, creating concerns for the sustainability of the burgeoning adalimumab biosimilar market.

An FDA advisory committee last year agreed that the data do not support phenylephrine as an effective nasal decongestant.

If approved, donidalorsen would be a first-in-class RNA-targeted medicine for hereditary angioedema. The agency’s goal date is August 21, 2025.
In the ESSENCE trial, semaglutide improved liver fibrosis in patients with metabolic dysfunction-associated steatohepatitis (MASH).

Committee members said there was uncertainty around sotagliflozin in patients with kidney disease. The FDA is currently reviewing the oral therapy as an adjunct to insulin to help control glycemic levels in adults with type 1 diabetes and chronic kidney disease. The agency’s goal date is Dec. 20, 2024.
ICER has given tabelecleucel a rating of A, indicating the T cell therapy for Epstein-Barr virus related post-transplant lymphoproliferative disease has a high certainty of substantial net health benefit and would be cost-effective if priced between $143,900 and $273,700.
Scemblix (asciminib) is a new first-line option for adults with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase (Ph+ CML-CP).
Regulators are also reviewing Teva’s application for its Prolia biosimilar candidate. The FDA’s decisions are expected mid-year 2025.

PTC Therapeutics' NDA resubmission of Translarna was based on data from Study 041 and the long-term STRIDE registry. No PDUFA action date was provided for regulators.

Prademagene zamikeracel (pz-cel) could be a new treatment for rare genetic disease recessive dystrophic epidermolysis bullosa (RDEB).

Qsymia’s manufacturer also released postmarketing data showing the oral therapy for weight loss was associated with reductions in 24-hour mean systolic blood pressure.

Gavreto used to treat metastatic RET fusion-positive non-small cell lung cancer and RET fusion-positive thyroid cancer.

This week’s approval expands Seldardsi indications to treat adults with Crohn’s disease and ulcerative colitis.
Zolbetuximab — now with the brand name Vyloy — is a monoclonal antibody to treat patients with advanced gastric and gastroesophageal cancers.

David Joyner has been appointed president and CEO of CVS Health effective immediately.
Imuldose will be marketed in the United States by Accord BioPharma and is expected to launch in the first half of 2025.