A new paper by the Institute for Clinical and Economic Review (ICER) looks at the cost-effectiveness versus affordability issue of obesity medications such as Wegovy and Zepbound.

A new paper by the Institute for Clinical and Economic Review (ICER) looks at the cost-effectiveness versus affordability issue of obesity medications such as Wegovy and Zepbound.
The FDA has set a goal date of Aug. 27, 2025. If approved, the therapy would be branded as Lytenava and be the first ophthalmic formulation of bevacizumab.

Bkemv is being offered at 10% off Soliris, and Epysqli is offered at 30% below Soliris. Both biosimilars treat several of the same rare immune diseases as Soliris.
Uplizna is the first approved treatment for immunoglobulin G4-related disease (IgG4-RD), a chronic inflammatory condition that can affect multiple organs. It has a price of $140,248.50 per dose.
Vanrafia reduces proteinuria in adults with primary immunoglobulin A nephropathy (IgAN). It has a wholesale acquisition cost of $162,500 annually.

The fourth pair of denosumab biosimilars, Conexxence and Bomyntra, are expected to launch in the United States in mid 2025, as a result of a global settlement with Amgen, according to a company news release.

Vykat XR will be available in April to treat the intense hunger that is a hallmark of the rare genetic disease Prader-Willi syndrome. The price is based on a patient’s weight, and the average patient in the clinical trials would have had an average annual cost of $466,200 for the first year.
Neuroendocrine tumors that start in the pancreas tend to be more aggressive and have a poor prognosis.
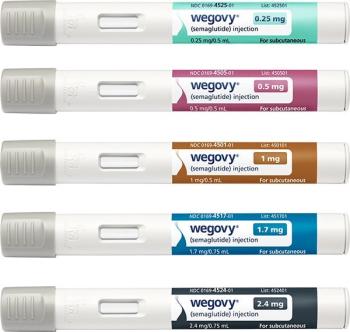
Wegovy is also available through NovoCare Pharmacy, which directly ship all dose strengths at a cost of $499 per month for cash-paying patients.

This expanded indication for Amvuttra makes it the first and only FDA-approved treatment for transthyretin amyloidosis with cardiomyopathy (ATTR-CM) and the polyneuropathy of hereditary transthyretin-mediated amyloidosis (hATTR-PN) in adults.

Tremfya is also approved to treat patients with plaque psoriasis, active psoriatic arthritis and ulcerative colitis and is available as both a subcutaneous and intravenous induction option.

Optum Rx's new cost-based program uses an internal algorithm that leverages multiple available industry data sources, such as NADAC and PAC, to inform reimbursement.

Optum Rx has eliminated reauthorization for almost 70 drugs for chronic diseases, about 10% of its overall pharmacy prior authorizations.

Regulators are expected to make a decision in the third quarter of 2025 on Kerendia to treat patients with heart failure with a left ventricular ejection fraction (LVEF) of ≥40%.

Steqeyma is one of seven Stelara biosimilars that have been approved by the FDA.

Lumicera Health Services, Navitus’ specialty pharmacy, has made a deal with Teva to offer an unbranded biosimilar that they estimate will save $112,000 and $336,000 per patient per year. Navitus will remove Stelara from formulary on July 1, 2025.

The new bill would also require state Medicaid programs to report National Average Drug Acquisition Costs (NADAC) as a way to increase transparency in drug pricing.
Two companies have received approved for a generic of the 2.5 mg tablet of anticoagulant rivaroxaban, which is used to reduce the risk of stroke and deep vein thrombosis.

Celltrion’s Omlyclo is interchangeable with Xolair to treat the same conditions: asthma, chronic rhinosinusitis, food allergy and chronic spontaneous urticaria.

Neffy 1 mg is now approved by the FDA to treat pediatric patients who weigh 33 to 65 lbs. Neffy was first FDA-approved as a 2 mg dose in August 2024 for the emergency treatment of anaphylaxis in children and adults weighing at least 66 lbs.

NovoCare Pharmacy will provide all dose strengths of Wegovy at a reduced cost of $499 per month for cash-paying patients without insurance.

The FDA has accepted Amneal’s biologics licensing application for two biosimilars referencing Prolia and Xgeva.
The FDA had approved Otulfi in September 2024 to treat Crohn’s disease, ulcerative colitis, psoriasis and active psoriatic arthritis. Information on pricing is not yet available.

The Institute for Clinical and Economic Review has prepared a special report on Trelegy and Breo, two therapies that treat patients with chronic obstructive pulmonary disease that are part of CMS's drug price negotiation program.

Selarsdi and Yesintek have launched this month at prices that are 85% and 90% off the cost of Stelara.