
Physicians in a new survey by the American Medical Association said prior authorization leads to delayed care and a high administration burden for physician practices.

Physicians in a new survey by the American Medical Association said prior authorization leads to delayed care and a high administration burden for physician practices.

Committee members, however, also said more data are needed on donanemab to treat patients in underrepresented patient groups, including Latin American and African American patients and special populations such as Down syndrome.
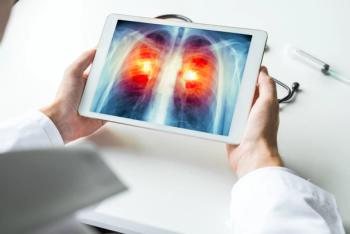
Tagrisso, which is already available to treat patients with non-small cell lung cancer and EGFR mutations, is being reviewed by the FDA for patients with this cancer and exon 19 deletions or exon 21 mutations.
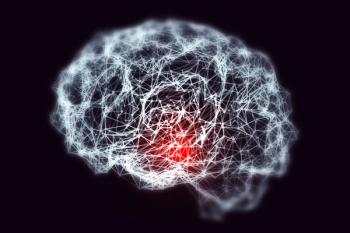
The FDA is considering a monthly IV maintenance dose of Leqembi to treat people with Alzheimer’s disease. The action date is set for Jan. 25, 2025.

Included in this review will be 11 drugs that ICER assessed for cost-effectiveness in 2022. New this year is an assessment for consumer accessibility of drugs, including the burdens of prior authorization and patient cost-sharing measures.

Vanity Fair shines a light on how fake Ozempic and other semaglutide products have found their way into the U.S. supply chain.

Patients with PTSD in the clinical trials of midomafetamine were able to guess whether they received treatment or placebo. Regulators and advisory committee members said this could have impacted efficacy results. FDA’s decision is expected by Aug. 11, 2024.

mRESVIA will be available for the 2024/2025 RSV season as a pre-filled, ready to use syringe.
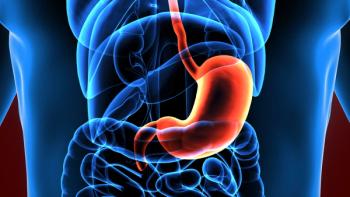
Zolbetuximab is first-in-class monoclonal antibody. The FDA has assigned an action date of Nov. 9, 2024.

LucyRx will begin providing pharmacy benefit services to self-insured employers, covering about half a million lives, beginning in January 2025.

FDA officials have asked for additional analyses on the efficacy of Dupixent in the two pivotal trials. The revised target action date is Sept. 27, 2024.
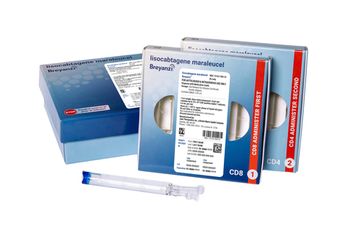
Breyanzi is a CAR T-cell therapy now approved for four subtypes of non-Hodgkin lymphoma.

Onyda XR is a liquid form of clonidine that can be used as monotherapy or as adjunctive therapy for patients 6 years of age and older with ADHD.
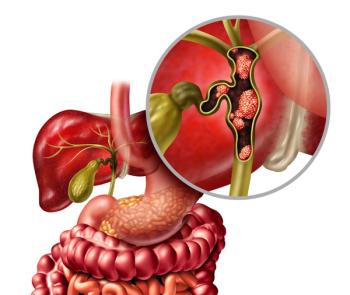
Zanidatamab is bispecific antibody that targets HER2 in patients with metastatic biliary tract cancer. The FDA has set an action date of Nov. 29, 2024.
The FDA has set a Prescription Drug User Fee Act date of Nov. 27, 2024, to treat patients with PIK3CA-mutated HR-positive, HER2 negative metastatic breast cancer.
Bkemv is a monoclonal antibody that is approved to treat two rare conditions that break down red blood cells.

In the United States, spending on oncology therapies rose to $99 billion in 2023, and is expected to grow to nearly $180 billion in 2028, finds a recent report by IQVIA Institute for Human Data Science.
Verastem is studying the combination of avutometinib and defactinib to treat low-grade serous ovarian cancer in patients with KRAS-mutations. The company plans to complete the new drug application in the second half of this year.
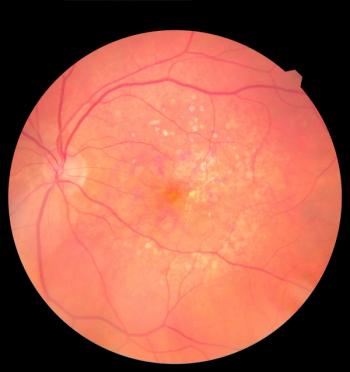
Yesafili and Opuviz are approved to treat patients with neovascular (wet) age-related macular degeneration, macular edema following retinal vein occlusion, diabetic macular edema and diabetic retinopathy.
The new program will launch in January 2025 and provide plan sponsors greater predictability of pharmacy spend.
If approved, tabelecleucel would be the first therapy specifically to treat Epstein-Barr virus related post-transplant lymphoproliferative disease.
Tarlatamab — now with the brand name Imdelltra — is the first approved bispecific antibody for a solid tumor.

Breyanzi is now included in the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology as a recommendation for third-line therapy for relapsed or refractory follicular lymphoma.
If approved, the Leqembi autoinjector could be used to administer the treatment for Alzheimer’s disease at home or at medical facilities.
Upstaza is a one-time gene therapy to treat patients 18 months and older with AADC deficiency. It has a review date of Nov. 13, 2024.

Boehringer Ingelheim also received FDA approval for a high-concentration formulation of Cyltezo, its branded Humira biosimilar.