
Cigna officials cite significant growth in Evernorth Health Services and Express Scripts for the increase in revenue.

Cigna officials cite significant growth in Evernorth Health Services and Express Scripts for the increase in revenue.

CVS Caremark is requiring step therapy through a generic prescription proton pump inhibitor before providing coverage.

Zunveyl, a prodrug of galantamine that addresses the gastrointestinal side effects, will be available in the first quarter of 2025.

Higher costs per claim, coupled with an increased number of patients using specialty drugs, has contributed to higher spend.

Leqselvi is approved to treat adults with severe alopecia. One-third of patients in clinical trials experienced 80% scalp hair coverage at 24 weeks.

Duvyzat was approved in March 2024 and works to reduce the inflammation and loss of muscle experienced by patients with Duchenne muscular dystrophy.
Epysqli is a monoclonal antibody that is approved to treat two rare conditions that break down red blood cells.
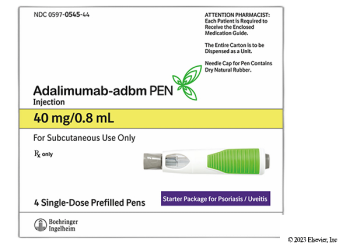
Boehringer Ingelheim’s high- and low-concentration adalimumab-adbm will be available through GoodRx for $550 for two-pack, which represents a 92% discount from the Humira list price.
If approved, tabelecleucel would be the first therapy specifically to treat Epstein-Barr virus related post-transplant lymphoproliferative disease. The FDA’s action date is Jan. 15, 2025.

OX124 is a nasal spray provides rapid absorption of naloxone for patients experiencing an opioid overdose. The FDA would like to see additional technical data, as well as data on whether patients can correctly use the device.

The switch to biosimilars helped Navitus clients offset increased utilization in the non-specialty category and the introduction of higher-cost specialty drugs.
Giant cell arteritis is an inflammatory disease that affects the large blood vessels that supply blood to the head and brain.
The FDA is asking for information about the manufacturing process, as well as the type 1 diabetes indication.

The FTC’s interim report on pharmacy benefit managers (PBMs) was just the latest effort to highlight what some say is an industry that profits at the expense of patients and independent pharmacists. The PBMs say the report paints an incomplete, misleading picture. Others say it shows the FTC is prepping its antitrust case.

The PBM industry said the FTC has not been objective, and that efforts to limit PBM negotiating tools would put patients at the mercy of drug manufacturers.
Genentech had recalled the Susvimo ocular implant two years ago. The FDA has approved changes to the implant and needle.
The prefilled syringe of Vabysmo will become available in the coming months. It treats age-related macular degeneration, diabetic macular edema and macular edema following retinal vein occlusion.
Company officials have said the bundled payment program could negatively impact sales of Xphozah, which was approved last year to reduce serum phosphorus in patients with kidney disease on dialysis.

Donanemab — now with the brand name of Kisunla — slows cognitive and functional decline by up to 35% and has a list price of $695.65 per vial.

Samsung Bioepis’ Pyzchiva (ustekinumab-ttwe) will be available beginning Feb. 22, 2025 and will be marketed by Sandoz.
For the second time, the FDA is asking for additional information about chemistry manufacturing and controls.
Ohtuvayre - an inhaled bronchodilator with non-steroidal anti-inflammatory effects - is the first new mechanism in COPD approved in more than 20 years.
Epkinly is a bispecific antibody now approved to treat both relapsed or refractory follicular lymphoma and relapsed or refractory diffuse large B-cell lymphoma.
The 3Axis Advisors analysis suggests PBMs’ spread pricing practices leads to employers being charged different amounts for the same medications and to pharmacists facing reimbursement challenges.

The FDA cited issues related to a third-party manufacturer. The agency did not request any additional testing from AbbVie.

The recalled batches have been found to have potassium chloride that does not dissolve, which can cause high potassium levels that can lead to hypertension, heart failure, or renal dysfunction.