
If approved, midomafetamine would be the first psychedelic-assisted therapy approved for any mental health condition. The advisory committee meeting is June 4, 2024.

If approved, midomafetamine would be the first psychedelic-assisted therapy approved for any mental health condition. The advisory committee meeting is June 4, 2024.

Zenocutuzumab is a new type of bispecific antibody that target both HER2 and HER3 proteins to inhibit NRG1 binding and blocking the mechanism for tumor survival. The FDA’s target date is in December 2024.

The Peripheral and Central Nervous System Drugs Advisory Committee will meet on Monday, June 10, 2024, to discuss the phase 3 trial of Lilly’s donanemab to treat patients with early symptomatic Alzheimer’s disease.
Over the next five years, list prices of protected prescription drugs are expected grow between 1% and 4% a year, while net prices are expected to decline by the same amount, IQVIA predicts.

Myhibbin is a ready-to-use mycophenolate mofetil oral suspension to prevent rejection of kidney, heart and liver transplants.

Bristol Myers Squibb is seeking approval of the subcutaneous formulation for all previous indications of Opdivo. The FDA has assigned a goal date of Dec. 29, 2024.

Specialty medications represent a large and growing proportion of overall drug spend, but plans and employers are struggling to understand their costs to make good decisions, finds the latest PSG Specialty Drug report.

The high-concentration biosimilar will be distributed under Quallent’s private label and will be available in June through Evernorth’s Accredo specialty pharmacy.
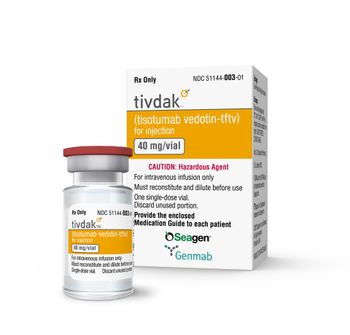
A phase 3 trials showed Tivdak demonstrated a 30% reduction in the risk of death. The U.S. list price is $6,599 per 40 mg single dose vial.

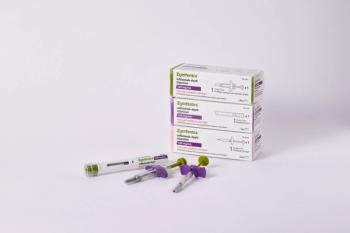
Zymfentra launched in March 2024 as the first subcutaneous formulation of infliximab for patients with ulcerative colitis and Crohn’s disease. It has a list price of $6,181.08 for two shots over four weeks.

Xolremdi is the first therapy for WHIM syndrome, which can cause recurrent lung infections and papillomavirus-related warts. It’s available in two doses: 400 mg for an annual cost of $496,400 and 300 mg for an annual cost of $372,300.

Libervant is film version of diazepam that is placed inside the cheek to treat children between 2 and 5 years of age who have refractory epilepsy.

Beqvez (fidanacogene elaparvovec) is priced at $3.5 million, which is on parity with Hemgenix, the first one-time therapy to treat adults with hemophilia B. Pfizer’s warranty will refund insurers and continue to provide coverage for patients if they change insurers.

Walgreens is expanding its specialty pharmacy services and renaming AllianceRx Walgreens Pharmacy as Walgreens Specialty Pharmacy.

Beginning in June 2024, Quallent Pharmaceuticals will provide Evernorth a high- and low-concentration interchangeable biosimilar of Humira.

Ojemda will have a wholesale acquisition cost of $33,916 for a 28 day supply and will be distributed through specialty pharmacies Biologics by McKesson and Onco360.

New White Paper looks at the solutions and policy options of how to pay for high-cost gene therapies when there is still so much uncertainty about the outcomes and the durability of clinical benefits.

Dr. Reddy’s is recalling five lots of Javygtor and one lot of generic sapropterin dihydrochloride because discoloration could lead to decreased potency. Sapropterin is used to treat an inherited metabolic disorder.

Lutathera is a radiotherapeutic that is now approved for patients 12 years and older neuroendocrine tumors in the pancreas or gastrointestinal tract.
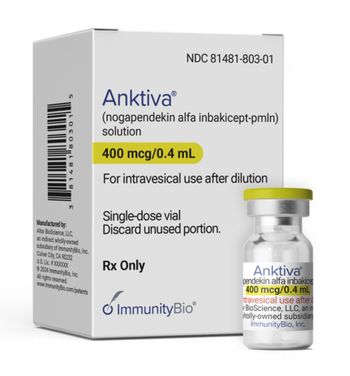
ImmunityBio’s Anktiva is an antibody cytokine fusion protein approved to treat patients with non-muscle invasive bladder cancer.

Prademagene zamikeracel is a cell therapy designed to incorporate the functional collagen-producing COL7A1 gene into a patient’s own skin cells. The FDA is asking for additional information on manufacturing practices.

An additional seven lots of Treprostinil are affected on top of last month’s recall of one lot.
Entyvio as an injection and subcutaneous formulations is approved to treat both Crohn’s and ulcerative colitis.

Alvotech’s Selarsdi (ustekinumab-aekn), a biosimilar referencing Stelara (ustekinumab), gained FDA approval, making it the second ustekinumab biosimilar and second for the company to be given the green light for the American market.

Researchers were unable to find a significant benefit for patients for the majority of cancer medications given accelerated FDA approval.