The Prescription Drug User Fee Act date is June 7, 2024, for the additional indication of preventing respiratory syncytial virus (RSV) in adults aged 50 to 59 who are at increased risk. It is already approved for those over the age of 60.

The Prescription Drug User Fee Act date is June 7, 2024, for the additional indication of preventing respiratory syncytial virus (RSV) in adults aged 50 to 59 who are at increased risk. It is already approved for those over the age of 60.
The FDA has set an action date of Nov. 29, 2024, to review acoramidis to treat patients with transthyretin amyloid cardiomyopathy.

The Oncologic Drugs Advisory Committee will meet on March 15, 2024, to review overall survival data for Abecma in earlier lines of treatment in relapsed or refractory multiple myeloma.

The bulk of Cigna’s $195.3 billion 2023 revenue comes from Evernorth. And Cigna is positioning itself to grow Evernorth even more.

The sale, valued at about $576.5 million, is part of Rite Aid’s restructuring plan after filing for bankruptcy in October 2023.
New research finds that in 2022, U.S. prices across all drugs were almost three times higher than in other countries.

Biogen will focus its resources on Leqembi and research of other treatments for Alzheimer’s disease.

Defender Pharmaceuticals is working with U.S. Naval Medical Research Unit and NASA to develop intranasal scopolamine for use in military personnel and astronauts.
Bristol Myers Squibb is seeking approval of Breyanzi, a CD19-directed CAR T-cell therapy, to treat patients with follicular lymphoma and mantle cell lymphoma.
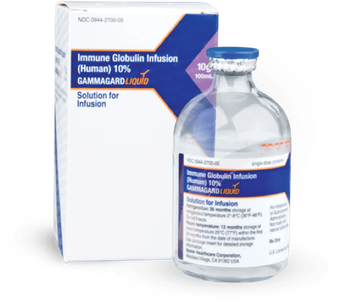
Gammagard is an intravenous immunoglobulin therapy now approved to improve neuromuscular disability and impairment in adults with chronic inflammatory demyelinating polyneuropathy.

Celltrion’s application is based on data from a phase 3 trial of patients with rheumatoid arthritis comparing its biosimilar with Actemra.
The FDA orders new warnings for CAR-T cell therapies due to reports of T-cell malignancies, including fatal cases, applying to BCMA- and CD19-directed genetically modified autologous T-cell immunotherapies.

The antihistamine drug carbinoxamine was found in one bottle Zenzedi, which is used to treat narcolepsy and ADHD.
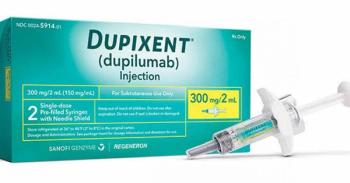
Eosinophilic esophagitis is progressive disease driven in part by type 2 inflammation. Dupixent is the first treatment for children as young as 1 year old.

CVS Caremark will continue to manage specialty drug benefits for Tyson Foods.

Joseph M. Shields talks about the formation of Transparency-Rx and its goals of achieving PBM reform that focuses on “corrective steps to address the misalignment in the marketplace to ensure that there’s competition and choice.”

Coverage of contraceptives and contraceptive care without cost-sharing is required under the Affordable Care Act.

Current treatments for urea cycle disorders, which causes ammonia to build up in the blood, have a bitter taste and smell. Pheburane was developed with a special coating that masks the taste of sodium phenylbutyrate. It has a list price of $4,375 per bottle.

Peter Rubin of No Patient Left Behind worries that patients may not see benefits, either in lowered costs or continued investment in new therapies, from drug price negotiations required under the Inflation Reduction Act.
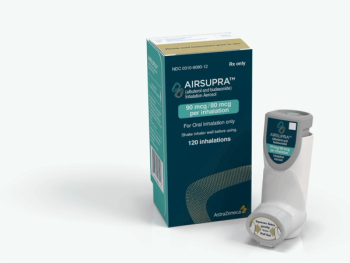
Airsupra is an anti-inflammatory rescue medication that can treat the symptoms of asthma while helping to prevent an attack.
Balversa targets FGFR3 genetic alterations and is approved as a second-line treatment for in adult patients with metastatic urothelial carcinoma.

Regulators are warning about the increased risk of very low blood calcium levels in patients with advanced chronic kidney disease.
Last year saw the approval of several firsts, including the first vaccines to prevent respiratory syncytial virus in older adults and infants and a first vaccine to prevent the mosquito-borne virus chikungunya.

A 2020 rule stands in which copay assistance from pharmaceutical companies counts toward deductibles for brand name drugs without a generic competition.

IQVIA’s most recent Global Use of Medicines report projected that market introductions of new biosimilar and generic drugs will increase projected losses for originator products from $111 billion to $192 billion over the next five years.

HyQvia is now available a maintenance therapy for adults with chronic inflammatory demyelinating polyneuropathy, which can lead to weakness and loss of feeling in the arms and legs.