Elacestrant, now with the brand name Orserdu, is an oral selective estrogen receptor degrader (SERD), which works by blocking the effects of estrogen on hormone receptor-positive breast cancer cells.

Elacestrant, now with the brand name Orserdu, is an oral selective estrogen receptor degrader (SERD), which works by blocking the effects of estrogen on hormone receptor-positive breast cancer cells.

Jaypirca is the first BTK inhibitor specifically approved for patients with mantle cell lymphoma previously treated with a covalent BTK inhibitor. It has a wholesale acquisition cost of $21,000 per 30 days of therapy.

This approval marks the fifth indication for Keytruda-based regimens in non-small cell lung cancer and the 34th indication in the United States.

AstraZeneca is developing a next-generation long-acting antibody for pre-exposure prophylaxis of COVID-19, which it plans to make available in the second half of 2023.

The Peripheral and Central Nervous System Drugs Advisory Committee meeting is scheduled for March 22, 2023, to review Biogen’s tofersen.

Once-daily, oral SGLT2 inhibitor Brenzavvy has been shown to reduce blood sugar and improve glycemic control.

A phase 3 trial showed that Jardiance reduced the risk of kidney disease progression or cardiovascular death in adults with chronic kidney disease by 28% compared with placebo. An FDA decision is expected in the second half of 2023.

Moderna plans to submit to the FDA its investigational mRNA vaccine, RNA-1345, for respiratory syncytial virus (RSV) in the first half of 2023.

Lilly expects topline results from the confirmatory phase 3 trial in the second quarter of 2023 and plans to submit for full approval.
This is the fourth indication for the Bruton’s tyrosine kinase inhibitor.
Tukysa with trastuzumab is indicated for second-line treatment of HER2-positive metastatic colorectal cancer, the first such therapy for this cancer.
There are currently no medications approved for the treatment of NASH, which can lead to cirrhosis, eventual liver failure, cancer and death. The FDA has assigned a PDUFA target action of June 22, 2023, for the therapy, obeticholic acid.

Rykindo is a bi-weekly long-acting risperidone injection to treat patients with schizophrenia and bipolar 1 in adults.

This update removes a previous limitation that it could not be used as the first therapy.

Airsupra is the first rescue medication to manage both the symptoms and the inflammation associated with asthma.

New approvals include gene therapies, first-in-class drugs, treatments for rare diseases, and cancer, as well as biosimilars.

Clarivate analysts have identified the treatments they forecast could deliver annual sales of more than $1 billion within five years. These include therapies for rare diseases, as well as HIV, Parkinson’s disease, Crohn’s disease, alopecia, and cancer.

The agency has assigned a Prescription Drug User Fee Act target action date of May 10, 2023, and plans to hold an advisory Committee
The supplemental BLA is based on data from a confirmatory phase 3 trial. The submission was made the same day Eisai/Biogen received accelerated approval.

Lecanemab, now with the brand name Leqembi, will launch with a wholesale acquisition cost of $26,500 a year.
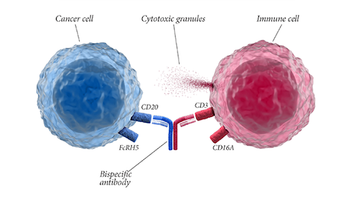
Genentech’s glofitamab is a bispecific antibody being reviewed to treat adults with large B-cell lymphoma. The FDA is expected to make a decision on approval by July 1, 2023.

Rozanolixizumab is a monoclonal antibody that addresses a different driver of generalized myasthenia gravis. An FDA decision is expected in the second quarter of 2023.
Nirsevimab is a single-dose, long-acting antibody to protect infants from respiratory syncytial virus (RSV) lower respiratory tract infections. The FDA action date is in the third quarter of 2023.

CT-P13 SC is a subcutaneous formulation of Celltrion’s Inflectra, a biosimilar of Remicade. The BLA seeks approval to treat patients with inflammatory bowel disease.

Wegovy is a once-weekly prescription medication for obesity that has faced supply issues. Its manufacturer, Novo Nordisk, is increasing production capacity in 2023.