
Cystic fibrosis drug Trikafta gets expanded indication for children ages 2-5.

Cystic fibrosis drug Trikafta gets expanded indication for children ages 2-5.

Uzedy’s wholesale acquisition cost (WAC) ranges from $1,232 to $3,080 per month.
CDI is a leading cause of infections acquired in healthcare settings, including hospitals and nursing homes. After recovering, individuals may get the infection again, and the risk of recurrence increases with each infection.

The list price for Qalsody, the first and only approved treatment to target a genetic cause of ALS, may prove to be controversial.

The expansion offers increased reimbursement opportunities and additional care services to rural independent pharmacies. In addition, it creates an Independent Pharmacy Advisory Committee to expand the role of rural, suburban and urban pharmacies in the healthcare system.
Omisirge is a stem cell therapy that helps the body recover more quickly from cancer treatments that kill normal blood cells along with blood cancer cells, thus lowering the risk of infection.
The FDA cited issues related to the proposed manufacturing of the drug, mirikizumab. Lilly expressed confidence that they could be corrected.
Through Express Scripts’ new Copay Assurance plan, consumers will have lower out-of-pocket costs because of caps on prescription drugs: $5 for generics, $25 for preferred brand drugs, and $45 for preferred specialty brand drugs.
Emergent BioSolutions hasn’t provided a price for the nonprescription Narcan but said the product will be available by late summer.
If approved, Perrigo’s Opill could be the first-ever nonprescription birth control pill.

Leniolisib, now with the brand name Joenja, treats APDA, a genetic disorder that impairs the immune system. It will have an annual list price of $547,500 a year.
Rezzayo is a novel once-weekly antifungal approved to treat invasive candidiasis, a serious infection that can affect the blood, heart and brain.

Regulatory officials identified additional requirements for approval of an extended-release version of Jakafi, which is under review for myelofibrosis, polycythemia vera and graft-versus-host disease.

Zynyx will be priced comparable to other PD-1 inhibitors currently available to treat metastatic or recurrent locally advanced Merkel cell carcinoma, a rare skin cancer.

Sandoz will now launch both high-concentration and low-concentration versions of Hyrimoz (adalimumab-adaz) in July.
In a complete response letter, the FDA has requested information about the pump that will be used to deliver ABBV-951, which will provide continuous subcutaneous delivery of oral immediate-release carbidopa/levodopa.

Evkeeza treats a rare form of high cholesterol. It is now approved for patients as young of 5 years of age.

Illuccix is for positron emission tomography (PET) imaging of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer.

Daybue will be available by the end of April with a list price of about $375,000 annually.

Zavzpret is the first calcitonin gene-related peptide (CGRP) receptor antagonist nasal spray approved for patients with acute migraine.

Other news includes the launches of the first generic of an ulcer drug, the antipsychotic lurasidone, an extended-release version of topiramate for migraine, and additional strengths of the cancer drug lenalidomide. Additionally, Glenmark will distribute Cediprof's generic Adderall, and the FDA has accepted Amneal’s application for a generic naloxone nasal spray.

If approved, Jardiance would be the first SGLT2 inhibitor indicated for this population. A decision is expected in the second quarter of 2023.

Voxzogo is already available to treat patients older than five years with a genetic cause of dwarfism. The FDA has set a PDUFA of Oct. 21, 2023, for children younger than 5.
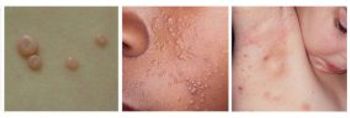
If approved, berdazimer could be the first prescription product treat molluscum, a common skin infection caused by poxvirus. The Prescription Drug User Fee Act goal date is Jan. 5, 2024.

With a Prescription Drug User Fee Act action date of Dec. 22, 2023, eplontersen is an antisense medicine to treat patients living with hereditary transthyretin-mediated amyloid polyneuropathy, which results in nerve damage.