
The FDA has requested an additional study. ARS Pharmaceuticals plans to appeal the decision.

The FDA has requested an additional study. ARS Pharmaceuticals plans to appeal the decision.

Atidarsagene autotemcel is a one-time gene therapy to treat patients with the rare and fatal disease metachromatic leukodystrophy (MLD). The agency has set a review date of March 18, 2024.

Lower out-of-pocket costs for patients might put upward pressure on drug prices, as manufacturers face less price sensitivity, note Matthew Majewski and Rhett Johnson of Charles River Associates. But they also note that upward pressure on price is likely to be limited to the inflation rate as any additional price increase would need to be paid back to CMS in the form of inflation rebates.
Earlier this year, RedHill Biopharma began a warranty program for patients taking Talicia, with a commitment to reimburse patient out-of-pocket costs if Talicia does not work.
Momelotinib — now with the brand name Ojjaara — is the first treatment for myelofibrosis patients with anemia. It has a list price of $26,900 for a bottle of 30 tablets.
Even though committee members voted in support of Onpattro for patients with cardiomyopathy related to transthyretin-mediated amyloidosis, there were questions about whether it provided a clinically meaningful benefit. The FDA set an action date of Oct. 8, 2023.
The date has been extended by three months to Feb. 24, 2024, because of resource constraints at the regulatory agency.
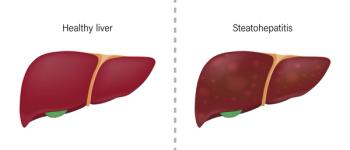
The FDA has granted priority review and assigned a review date of March 14, 2024, for resmetirom treat adult patients with nonalcoholic steatohepatitis (NASH) who have liver fibrosis.

Cell and gene therapies can be used for patients with cancer and serious diseases who have limited or no treatment options — but at a cost.
In clinical trials, Aphexda plus filgrastim enabled a majority of patients to achieve the collection goal to enable stem cell transplantation for patients with multiple myeloma. It will have a list price 5,900 per vial, with most patients needing two vials for treatment.
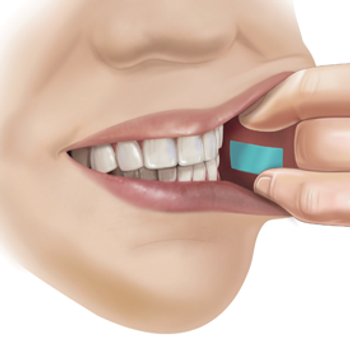
Libervant is film version of diazepam that is placed inside the cheek to treat children between two and five years of age who have refractory epilepsy. The PDUFA target goal date is April 28, 2024.

If approved, mavorixafor would be the first therapy to address the genetic defect that results in WHIM syndrome, an ultra-rare disease that can cause recurrent lung infections, papillomavirus-related warts, and an increased risk of developing certain types of cancer.
With this tentative approval of abacavir/dolutegravir/lamivudine, Viatris aims to help adherence to HIV treatment in low- and middle-income countries.
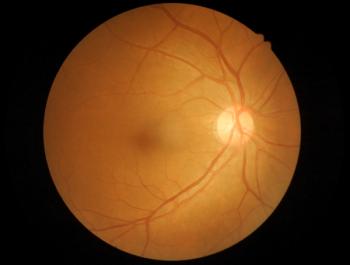
The anti-cancer drug bevacizumab is used off-label to treat wet AMD. Outlook Therapeutics had developed an ophthalmic formulation of bevacizumab for intravitreal injection.

At least 15 manufacturers have received approval for both capsule and chewable tablet generic versions of Vyvanse.
In head-to-head study, Reblozyl nearly doubled the percent of patients achieving primary endpoint of concurrent transfusion independence and hemoglobin increase vs. epoetin alfa.
The FDA granted accelerated approval to Balversa in 2019. Patients who received Balversa in Janssen’s confirmatory study achieved a median overall survival of more than one year. It reduced the risk of death by 36%.

The submissions to both the FDA and the EMA are supported by two phase 3 trials demonstrating Skyrizi achieved the primary endpoint of clinical remission. Skyrizi is already available to treat patients with plaque psoriasis, psoriatic arthritis, and Crohn’s disease.
Sandoz’s Tyruko, the first biosimilar to Biogen’s Tysabri, is expected to be available as soon as possible. Pricing has not yet been made available.
If approved, Xtandi would be the first hormonal therapy for non-metastatic patients with increasing PSA levels. The FDA has assigned an action date in the quarter of 2023.

The FDA set an action date of June 16, 2024, for imetelstat to treat transfusion-dependent anemia in myelodysplastic syndromes. Regulators said they plan to hold an advisory committee meeting as part of their review.

Abrysvo is the first RSV vaccine approved to be given to pregnant women at 32 to 36 weeks to prevent infection in infants.
In addition to tardive dyskinesia, Ingrezza now also treats chorea associated with Huntington’s disease, which is a movement disorder that effects coordination, gait, swallowing and speech.
The higher dose of Eylea allows longer intervals between injections for patients with macular degeneration and diabetic retinopathy. It will have a list price of $2,625 per single-use vial.

Veopoz is the first treatment for CHAPLE disease, which causes damage to blood and lymph vessels along the upper digestive tract. The list price is $34,615.38 per single-use vial.