
Winter exacerbates atopic dermatitis in children, but effective management strategies and new treatments offer hope for improved skin health and comfort.

Winter exacerbates atopic dermatitis in children, but effective management strategies and new treatments offer hope for improved skin health and comfort.

Here’s what you missed this week on Managed Healthcare Executive.
Discover groundbreaking advancements in dermatology from the 2025 Fall Clinical Conference, featuring innovative treatments for psoriasis, alopecia, and atopic dermatitis.
Pfizer’s sigvotatug vedotin combined with Keytruda is being studied in patients with non-small cell lung cancer. Researchers suggest sigvotatug vedotin’s ability to induce immunogenic cell death may enhance antitumor activity.

To gain approvals, biosimilar manufacturers could do faster, less expensive analytical studies of their products' composition.

The medical director of The US Oncology Network discusses adherence to clinical pathways, CAR-T therapy and concerns about the consequences of drug price negotiation under the Inflation Reduction Act.

The Residual Transformer Generative Adversarial Network (RRTGAN) generates high-quality, cellular-level retinal images from minimal data inputs, overcoming the limitations of previous slow and burdensome imaging methods.

The findings challenge the conventional view that daytime sleepiness is always a result of insufficient sleep, poor sleep hygiene or classic sleep disorders.

In 2025, atopic dermatitis care evolved with new treatments, prioritizing holistic methods and personalized strategies for improved patient outcomes.
Researchers used nanoparticle technology to encapsulate paclitaxel and target bladder tumors more effectively. Early results demonstrate tumor elimination and minimal side effects.

Kyverna’s investigational CAR-T therapy, mivocabtagene autoleucel (miv-cel) showed clinically meaningful mobility improvements in most stiff-person syndrome patients, prompting the company to plan an FDA BLA submission in 2026.
The new radiotracer takes advantage of two biological targets linked to fibrotic and inflammatory processes in the lung: fibroblast activation protein and integrin receptors.

Falls among older adults with visual impairment are strongly linked to modifiable home hazards—especially broken flooring and tripping risks—while fall risk remains relatively low in hazard-free homes.
Trial results presented at the annual meeting of the American Society of Hematology showed decided advantages of Hympavzi (mastacimab) over on-demand treatment with bypassing agents.
Despite widespread awareness of shingles risk factors, fewer than one-third of U.S. primary care physicians are fully aware of current ACIP shingles vaccine recommendations, highlighting the need for improved education and implementation.

The top stories out of the AAD annual meeting highlight how dermatology care is evolving.

The results of an American Society of Health-System Pharmacists survey shed some light on what is on the minds of pharmacy leaders at health systems.

A new influenza strain could fuel a severe flu season while a surge in measles cases threatens U.S. elimination status, emphasizing that vaccination remains critical to limiting disease spread and severity, according to faculty from Johns Hopkins Bloomberg School of Public Health.

Enrollment in Affordable Care Act health plans has soared because of enhanced subsidies enacted during the COVID-19 public health emergency. Democrats (and perhaps some moderate Republicans) want them continued. Republicans say they have better ideas about how to keep Americans in the individual market covered, including health savings accounts and association health plans.
Ophthalmologists and oncologists created a new ocular adverse event grading scale for oncology trials that addresses ambiguities and provides clear dose modification recommendations.

Addyi is the first FDA-approved treatment for low libido in women ages 65 and younger.
Dermatoscopy identifies visual features that predict melanoma metastasis risk, performing comparably to current pathology-based staging methods.

By 2020, only 10.8% of adults aged 50 and older had completed both doses of the recombinant zoster vaccine,with 14.1% receiving at least one.
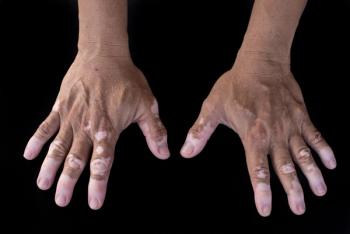
A study reveals that vitiligo significantly impacts quality of life and mental health in Japanese patients, highlighting the need for early psychological support.

The biosimilar market experienced 16 new approvals by early December 2025, marking significant expansion and introducing previously unavailable biosimilar drug classes to patients.
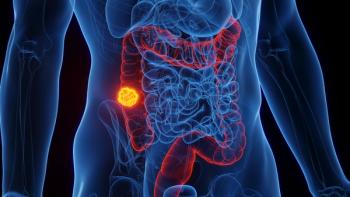
An analysis of patients with stage III colon patients found celecoxib improves survival only in those who are positive for circulating tumor DNA.

Teledermatology revolutionized skin care in 2025, enhanced access and efficiency through virtual tools and AI while addressing safety and trust concerns.
A study of more than 1 million UK medical records found that getting a flu vaccine between ages 40 and 50 did not increase Parkinson’s disease risk and was even associated with a lower estimated prevalence eight years later, suggesting a possible protective effect that warrants further research.

Medicare's first negotiated drug prices, effective January 2026, provide modest discounts, with limited savings for most beneficiaries and some unintended consequences.