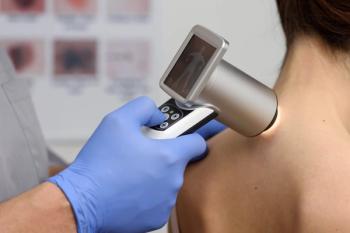
Dermatoscopy identifies visual features that predict melanoma metastasis risk, performing comparably to current pathology-based staging methods.

Dermatoscopy identifies visual features that predict melanoma metastasis risk, performing comparably to current pathology-based staging methods.

Flipped dosing of Yervoy and Opdivo produced a 49% response rate compared with 37% for the standard dose of the combination and extended survival to 42 months from 14 months in a Swedish study.

The Melanoma Research Alliance launched a national biorepository at the University of Colorado to collect melanoma samples, focusing on rare subtypes for diagnostic and treatment research.

The patch measures distinct electrical patterns, which indicates how easily electrical signals pass through living tissue. Cancerous areas have different electrical properties than healthy skin.
A phase 1 trial highlights the role the microbiome plays in resistance to checkpoint inhibitors, as well as to immune-related adverse events from treatment.

UCLA study reveals why melanoma returns after immunotherapy: tumors delete genes triggering cell death while copying survival genes. This understanding could potentially enable new combination treatments to prevent resistance.
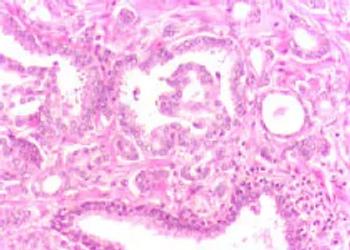
An NYU-led study found Keytruda immunotherapy after surgery lowered the risk of Merkel cell carcinoma spreading to the liver, lungs and bones.
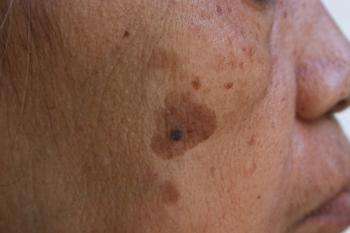
In a viewpoint column, some experts are suggesting that melanoma in situ, which generally doesn’t lead to invasive melanoma, as a precancer to reduce unnecessary biopsies, surgeries, and emotional distress among patients.
An engineered CD40 antibody that can bind to a specific immune receptor and directly injected into tumors has demonstrated in a phase 1 trial that it can trigger a systemic immune response.


Research shows older adults had 4.45 million skin cancer cases in 2021. Basal cell carcinoma is expected to double by 2050 worldwide.

In a large retrospective study of veterans, the B3 derivative nicotinamide was found to be effective at reducing the risk of squamous cell carcinoma.

Gen Z is most at risk for skin cancer due to a lack of knowledge about the sun and how to protect themselves.
A dissolvable microneedle patch delivering doxorubicin shows encouraging efficacy in a phase 2 trial to treat patients with basal cell carcinoma.

Researchers from the University of Michigan have developed a skin patch that was able to accurately detect melanoma in tissue samples from mice.
A recent study reveals that 89% of patients with unresectable desmoplastic melanoma respond to Keytruda.
IO Biotech plans to meet with the FDA this fall to discuss the data from the phase 3 trial of Cylembio and determine next steps for a potential biologics license application submission.

CMS is brokering outcomes-based agreements on behalf of Medicaid programs. Thirty-three states, along with Washington, D.C., and Puerto Rico, have signed on, collectively covering about 84% of Medicaid enrollees with sickle cell disease.
The response rate to Amtagvi was higher in patients who had received two or fewer therapies than in those with more heavily treated disease.

Studies show in mice show that targeting the metabolic pathways could inhibit the growth of melanoma and combining this strategy with other treatments could enhance the therapeutic effect.

All 10 participants in this trial showed improvements in hearing within the first month of receiving the gene therapy, which delivered healthy copies of the OTOF gene.

The eavesdropping artificial intelligence saves physicians time and means more eye contact during patient visits.


FDA officials have determined that information regarding the risks for six CAR T-cell therapies can be communicated through product labeling, which includes a boxed warning for the risks of cytokine release syndrome and neurological toxicities.

Most gene therapies being studied for eye diseases are in earlier phases of development, and a third of the gene therapies being investigated are for age-related macular degeneration.

When available, the educational toolkit will provide patients with sickle cell disease information on the gene therapy process, what to expect from therapy, and the importance of follow-up care.

EGFR inhibitors such as Erbitux and Gilotrif show potential in laboratory and mouse studies in treating melanoma with a specific mutation that can lead to resistance to immunotherapy.

AI apps that can detect skin cancer can be manipulated and potentially miss cases of melanoma, a serious skin cancer.

While heritable human genome editing is banned in the United States, across Europe and much of the world, the technology raises concerns about potential misuse.

Several oral abstracts presented at the American Society of Clinical Oncology’s annual meeting highlight ongoing research of Keytruda, Opdivo, Tecentriq and more in skin cancer.

Published: October 10th 2024 | Updated:

Published: November 18th 2024 | Updated:

Published: July 11th 2025 | Updated:
Published: November 18th 2024 | Updated:

Published: July 11th 2024 | Updated:
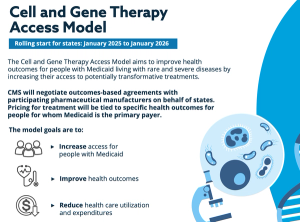
Published: January 31st 2025 | Updated: