
Jardiance demonstrated a 21% relative risk reduction for the composite primary endpoint of cardiovascular death or hospitalization for heart failure.

Jardiance demonstrated a 21% relative risk reduction for the composite primary endpoint of cardiovascular death or hospitalization for heart failure.


Officials with OptumRx predict four new therapies that are expected to an impact on patients and on the market.

Point32Health is the name of the not-for-profit health care organization formed by the merger of Harvard Pilgrim Health Care and Tufts Health Plan.

Investigators found patients with COPD taking Anoro Ellipta had low overall on-treatment exacerbation rates, which can reduce medical costs due to increased adherence to therapy.
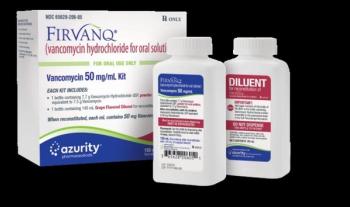
The lot contains kits with the incorrect solution for diluting vancomycin, which could lead to doses above or below recommended levels.

Investigators find there is strong adherence soon after patients fill prescriptions but less consistent use after initial treatment.

ICER’s updated analysis uses observational, real-world data for three therapies — Takhzyro, Haegarda, and Cinryze.
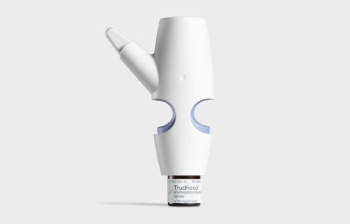
The therapy, to be launched in October, is delivered quickly to the bloodstream by targeting the upper nasal space.

The CDC and the FDA have updated their recommendations for the 2021-2022 flu season.

American Academy of Sleep Medicine’s guideline recommends Wakix to treat excessive daytime sleepiness and cataplexy in adults with narcolepsy.

The FDA’s review of the JAK inhibitor class concluded there is an increased risk of serious heart-related events such as heart attack or stroke, cancer, blood clots, and death with these arthritis and ulcerative colitis medicines.

The product is considered “super potent” with a higher dose that could result in serious cardiac toxicities.
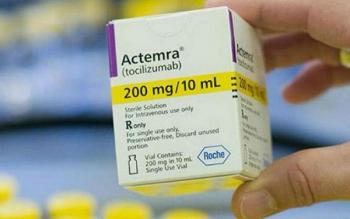
Pharmacists are referring patients elsewhere or are shifting patients who were using the IV form to the subcutaneous injection. The NIH has also recommended using Kevzara as an alternative where there are shortages of Actemra.

Investigators says Janssen’s Invega Hafyera offers longer-term symptom control with the fewest doses per year, which may support greater patient adherence.

The approval follows the FDA’s Oncologic Drugs Advisory Committee voting 5-3 in favor of maintaining the approval despite a confirmatory trial that found Keytruda did not meet the end points of overall survival and progression-free survival.
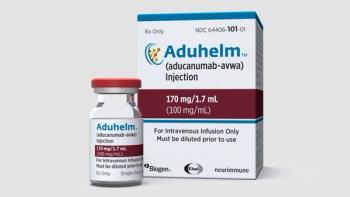
Florida’s First Choice Neurology has provided Aduhelm to patients through the Biogen access program.

The full results from the Emperor-Preserved trial show Jardiance reduces the risk of hospital and death from heart failure.

The combination bamlanivimab and etesevimab can resume use and distribution to treat mild-to-moderate COVID-19 but only in states where variants resistant to the therapy are low.
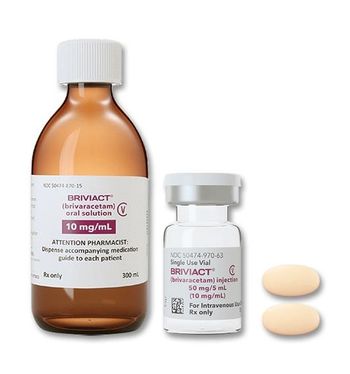
Tablets, oral solution, and IV dosage forms are now approved as both monotherapy or adjunctive therapy for the treatment of partial-onset seizures in patients one month of age and older.

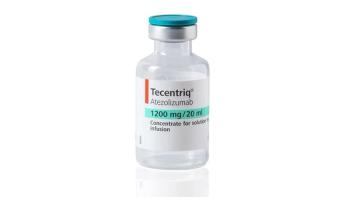
Tecentriq did not meet the primary end point in a postmarketing study as a first-line treatment.
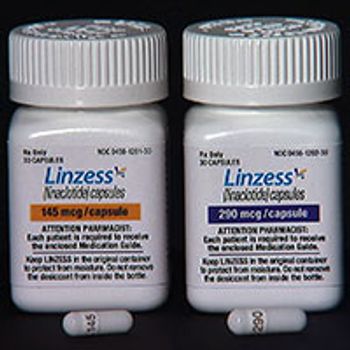
The update indicates there is a serious risk of dehydration and is contraindicated in children.

Bluebook Rx is a new solution that helps employers and plan sponsors better understand and optimize their prescription drug spending.

Tibsovo is the first targeted therapy for IDH1-mutated cholangiocarcinoma, a cancer of the bile ducts within and outside the liver.