Formulary Watch
Latest News
All News


Amgen’s Biosimilar Trend Report finds that biosimilars have been introduced at a price that is generally 15% to 37% lower than the reference product.

Shifting cancer patients to the physician setting for administration of cancer therapies could save insurers $1.28 billion.

Takeda Oncology’s Exkivity treats non-small lung cancer in patients with EGFR exon 20 insertion mutations.

This is the fourth approval for Jakafi, a JAK1/JAK2 inhibitor.

Keytruda plus chemotherapy reduces the risk of death by 27% in patients with triple-negative breast cancer.

People over the age 65 and those at high risk of severe COVID-19, including health care workers and those with occupational exposure, are eligible for the booster.

Dr. Reddy’s has also launched a generic of Librax. Recent generic approvals include clindamycin phosphate foam and sodium acetate.

Opzelura is the first and only topical formulation of a JAK inhibitor approved in the United States.
Tivdak is the first approved antibody-drug conjugate for the treatment of adult patients with recurrent or metastatic cervical cancer.

In trials, Skyrizi demonstrated improvements in clinical remission and endoscopic response as both induction and maintenance therapy

The FDA has set a Prescription Drug User Fee Act (PDUFA) goal date of March 19, 2022.

Biogen/Samsung Bioepis’ Byooviz is a biosimilar of Genentech’s Lucentis.
Effective October 1, 2021, CMS will reimburse for the infusion product when dispensed in the outpatient setting.
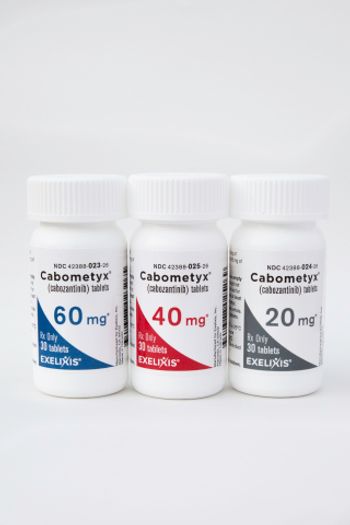
The approval comes more than two months ahead of its PDUFA target date of December 4, 2021.


The vaccines advisory committee voted late on Friday, September 17, 2021, to provide emergency authorization of Pfizer’s COVID-19 vaccine as a booster for those over the age of 65, as well as for those at high risk of developing severe disease.

Rinvoq achieved primary and secondary end points compared with placebo.

To date, Pfizer has not received reports of adverse events related to this recall, but is making the move as a precaution.
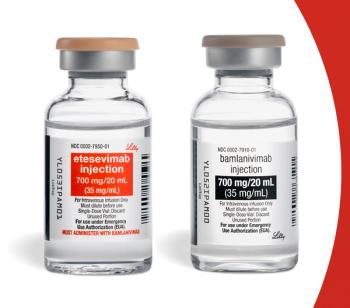
The combination of bamlanivimab/etesevimab can now be given to both treat and prevent COVID-19 infection after exposure.
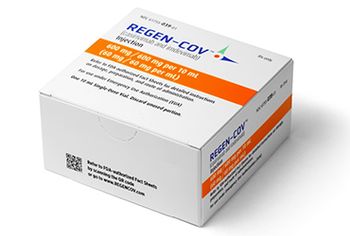
Regeneron also announced an expanded contract with the U.S. government for the purchase of additional doses of REGEN-COV.

Takeda Oncology’s Exkivity treats non-small lung cancer in patients with EGFR exon 20 insertion mutations. This is the second approval for this mutation and the first oral therapy.
The FDA has set a Prescription Drug User Fee Act target action date of April 30, 2022.
The agency has granted accelerated approval to Brukinsa as a second-line treatment for patients with marginal zone lymphoma, a group of slow-growing non-Hodgkin lymphomas.

The plan will cover administration of the booster for those with cancer or who are immunocompromised.