Patricia Weiser, Pharm.D.
Articles by Patricia Weiser, Pharm.D.

It is well-known that smoking is linked to a higher risk of cancer, and those who smoke can lower their risk by quitting. However, it remains unclear exactly how many years of continued smoking cessation are needed to see a substantial risk reduction.

A study published in The Lancet revealed the efficacy of a single-dose typhoid conjugate vaccine. The four-year, phase 3 trial assessed the vaccine's effectiveness in preventing typhoid fever in Malawian children.
The FDA orders new warnings for CAR-T cell therapies due to reports of T-cell malignancies, including fatal cases, applying to BCMA- and CD19-directed genetically modified autologous T-cell immunotherapies.

The FDA orders new warnings for CAR-T cell therapies due to reports of T-cell malignancies, including fatal cases, applying to BCMA- and CD19-directed genetically modified autologous T-cell immunotherapies.



Invivyd‘s request for EUA is supported by positive initial results from the CANOPY Phase 3 clinical trial of VYD222.
To date, two shingles vaccines have been marketed in the United States: Zostavax, approved in 2006, and Shingrix, approved in 2017. However, Zostavax is no longer available for treatment.

While tissue biopsy is currently recommended as the gold standard for determining mutations in non-small cell lung cancer, its success is not guaranteed, prompting the consideration of the liquid biopsy method as a viable alternative.
COVID-19 vaccines are currently administered intramuscularly. Studies indicate that intramuscular vaccine administration may not effectively generate antibodies and T cells against SARS-CoV-2 in the respiratory tract mucosa.

Trial results revealed that tumors shrank in 40% of patients who received the 10-mg dose of tarlatamab and in about 32% of those who received the 100-mg dose.

This study examined data from 589,722 individuals and found that those who were vaccinated had a lower incidence of long COVID compared to those who were unvaccinated.
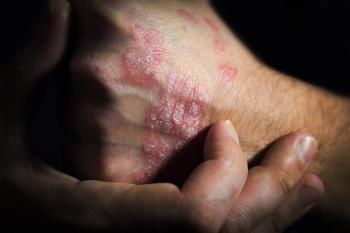
Vtama is a steroid-free cream that works by targeting the aryl hydrocarbon receptor, which plays a role in regulating immune responses.

Despite its efficacy, PrEP remains underutilized compared to the need for it. High costs are among the barriers to PrEP use, along with limited knowledge among clinicians, lack of health insurance, stigma, and underestimation of personal HIV risk.
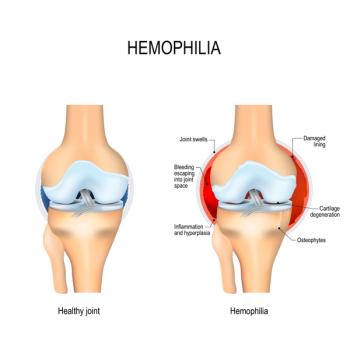
People with moderate or severe hemophilia had worse joint status, lower pain thresholds, and poorer quality of life compared with people with mild hemophilia or healthy controls.


Pfizer and BioNTech released positive top-line data for their mRNA-based combination vaccine that targets influenza and COVID-19.

Results of this prospective cohort study fits with other evidence showing no association between COVID-19 vaccination and miscarriage.

Recent data found that extreme heat is projected to lead to a significantly higher burden of excess cardiovascular deaths in the United States by midcentury (2036–2065), with elderly adults and non-Hispanic Black adults being most affected.

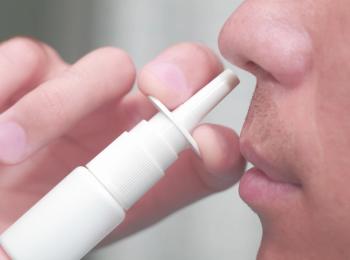
The self-administered nasal spray may increase vaccination rates.

The mean lag time between FDA approval and insurance coverage for 89 cancer drugs ranged from 2.3 months to 8.2 months. But cancer drugs approved more recently were more likely to have shorter coverage lags.
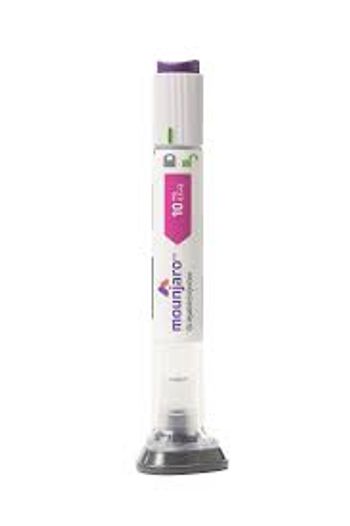
Patients taking Mounjaro after a lifestyle intervention saw a weight reduction of almost 20%.


Coadministration of flu and COVID-19 vaccines is safe and effective, according to findings published recently in JAMA Network Open, and Moderna report a strong immune response to its experimental vaccine that would combine flu and COVID-19 vaccination into a single shot.





























