Regeneron’s Libtayo FDA Approved as First Immunotherapy to Reduce Recurrence of High-Risk Squamous Cell Skin Cancer
Libtayo gained FDA approval as the first immunotherapy for high-risk cutaneous squamous cell carcinoma, promising improved outcomes post-surgery and radiation.
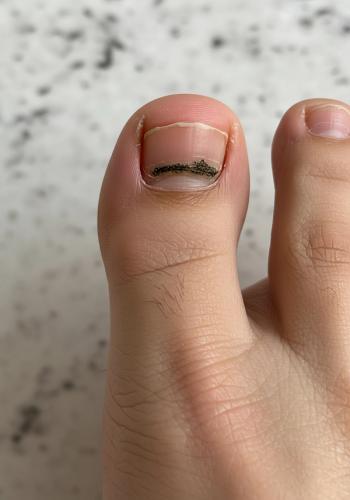
The FDA has approved a new use of PD-1 inhibitor Libtayo (cemiplimab-rwlc) as a treatment for cutaneous squamous cell carcinoma (CSCC) that has a high risk of recurring after it has been treated with surgery and radiation. The approval makes Libtayo the first immunotherapy approved for use after surgery and radiation and may change how patients are treated earlier in their disease.
As a PD-1 inhibitor, Libtayo targets receptors on T cells and, in doing so, unleashes them so they recognize and attack cancer cells. The FDA had previously approved the monoclonal antibody as a treatment for metastatic and locally advanced CSCC, metastatic and locally advanced basal cell carcinoma (BCC) and certain types of non-small cell lung cancer. Libtayo, manufactured by Regeneron, is also being studied for other cancers.
CSCC is one of the most common forms of skin cancer in the U.S., with roughly 1.8 million cases diagnosed annually. Surgery is a cure for most cases, but the disease recurs in a subset of cases.
“Patients whose CSCC is at a high risk of recurrence following surgery and radiation often have the poorest outcomes. Until now, we lacked options to help prevent a devastating recurrence, and immunotherapy was only available for patients with advanced CSCC who were no longer candidates for curative surgery or curative radiation,” Vishal A. Patel, M.D., associate professor of dermatology and hematology/oncology at George Washington University, and director of the GW Cancer Center’s Cutaneous Oncology Program,
Israel Lowy, M.D., Ph.D., clinical development unit head of oncology at Regeneron, said the approval was an important treatment advance for patients with CSCC. "As the first and only immunotherapy approved in the adjuvant setting, Libtayo offers a potentially practice-changing advancement for doctors to treat this patient population," Lowy said in an interview with Managed Healthcare Executive.
The FDA approval was based on results from the 3 C-POST trial, a phase 3 trial that compared Libtayo with placebo in patients at high risk of recurrent CSCC. The results of the study were published in the New England Journal of Medicine and presented at the 2025 ASCO Annual Meeting.
The trial results showed that
The trial also showed fewer local, regional and distant recurrences in the Libtayo group, highlighting its ability to prevent clinical disease events in a population with otherwise limited post-surgical options. More patients in the Libtayo group experienced serious side effects than in the placebo group (23.9% vs. 14.2%), and the discontinuation rate because of side effects was significantly higher in the Libtayo group compared with the placebo group. (9.8% vs. 1.5%).
The 3 C-Post researchers used nodal and nonnodal criteria for categorizing patients as having CSCC that is more likely to recur after surgery and postoperative radiation and therefore make them eligible for participation in the trial. The nodal criteria included having extracapsular extension with at least one node measuring 20 millimeters in diameter or three involved nodes regardless of extracapsular extension, according to the description of the trial that was part of the results reported in the New England Journal of Medicine. The nonnodal criteria included in-transit metastases, perineural invasion, T4 lesion or local recurrence. Approximately 60% of the study participants met the nodal criteria and approximately 40% met the nonnodal criteria.
Libtayo has also demonstrated clinical effectiveness and a favorable safety profile in other cancers, including advanced non-small cell lung cancer, where studies have shown
As of October 1, 2025,
Regeneron mentioned in its news release about the CSCC approval that it had launched Libtayo Surround to provide financial and educational support to patients.
Newsletter
Get the latest industry news, event updates, and more from Managed healthcare Executive.