HPV vaccine uptake increased almost 20% under guidance from the clinic-based program.

HPV vaccine uptake increased almost 20% under guidance from the clinic-based program.

Discrimination, fear and lack of knowledge are just a few of the obstacles they face when trying to access healthcare.

The American Thoracic Society (ATS) has recommended moving to race-neutral equations for assessing what lung functions mean. A study presented at the ATS meeting this weekend projected how the new equation would affect disease classification, lung test standards for becoming a firefighter and VA disability benefits.

Results from a phase 2a study suggest that the benefits of Tezspire (tezepelumab) for people with chronic pulmonary obstructive disease may be limited to those with elevated blood eosinophil counts indicative of type 2 inflammation.

Breast cancer stats from the American Association for Cancer Research 2024 Cancer Disparities Report.

A new study in JAMA Network Open suggests that disparities in outcomes between Black and White children with acute myeloid leukemia may be due to differences in pharmacogenomics, which is how genes affect drug responses.
Results from the phase 3 NOTUS trial presented at the ATS meeting confirm benefits of Dupixent (dupilumab) among 20% to 40% of people with chronic obstructive pulmonary disease (COPD) who have evidence of type 2 inflammation.
Pamrevlumab is the third investigational agent to fail in a phase 3 trials. Researchers said It may be time to stop treating idiopathic pulmonary fibrosis as a single disease and treat subcategories organized by relevant biomarkers.

The update was prompted by a recent increase in breast cancer cases among women in their 40s.

The FDA has approved a new type of bispecific antibody to treat small cell lung cancer and an additional indication for Breyanzi for patients with follicular lymphoma. The agency has set review date for gene therapy for enzyme deficiency. In addition, Biogen have Eisai hve begun a rolling submission of subcutaneous Leqembi for Alzheimer’s disease.

William Flanary, M.D.., has a huge social media following as Dr. Glaucomflecken, a character he created on Twitter when he was an ophthalmology resident in Iowa. More than 13,400 people have registered to attend the American Thoracic Society (ATS) International Conference, which is being held this weekend at the San Diego Convention Center.

Debra Boyer, M.D., M.H.P.E., discussed the program of the 2024 American Thoracic Society International Conference in San Diego and the limits of artificial intelligence.

Set to launch this summer, Uber Caregiver is tailored specifically for caregivers and their loved ones, aiming to tackle challenges head on and enhance the overall care experience.

According to findings collected by Cedars-Sinai, artificial intelligence can reduce serious health risks associated with pregnancy and childbirth, and improve screening for some gynecological cancers.

But the waivers that allow for coverage of tele-health expire at the end the year. Several bills have been put forth to extend coverage.
The authors of an article about relatively low biosimilar use by people with inflammatory bowel disease have suggestions for how to mitigage the nocebo effect.

The efforts to prevent one disease increased another, likely due to the lack of methadone and sterile needles.
The Biotechnology Innovation Organization has some suggestions, including "platform designation" by FDA and changes at the CDC.
The FDA has approved six with dermatologic indications and more are in late-stage trials.

Home testing for fecal calprotectin, a commonly used marker in IBD diagnosis, and symptom quetionnaires might hasten the diagnosis of inflammatory bowel disease.

Enrollment has soared, but a relatively modest 2025 rate increase and changes to the Star rating system may lead plans to cut back on with their benefit offerings.

Crenolanib may have advantages over other therapies targeting acute myeloid leukemia with FLT3 gene mutations.

This month's episode of the Meet the Board podcast features a new member of our editorial advisory board, Marci J. Chodroff, MD, FACP, vice president of Medical Affairs at Magellan Rx Management, a Prime Therapeutics company.
Darzalex (daratumumab) is a monoclonal antibody drug currently approved for both refractory and new multiple myeloma. It’s available in intravenous and subcutaneous formulations.

Over the next five years, list prices of protected prescription drugs are expected grow between 1% and 4% a year, while net prices are expected to decline by the same amount, IQVIA predicts.

The splashy meeting with more than 7,000 people in attendance reflects the growth of the specialty pharmacy sector.

Although chronic respiratory diseases remain the third leading cause of mortality, they have received less attention from the global community.
Persisting stigma and COVID loneliness are partly to blame.

The FDA made several approvals this week, including converting Tivdak’s accelerated approval to full approval for cervical cancer and approvals for a high-concentration formulation of Cyltezo, Xolremdi for an ultra rare immune disorder and Libervant Film for epilepsy in young children.
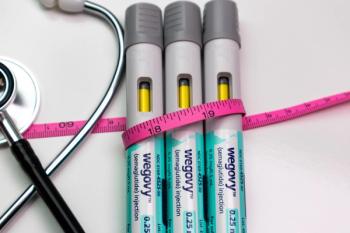
Health plans that use Optum Rx as their pharmacy benefit manager (PBM) will be allowed to tier their coverage of GLP-1s and still receive rebates under an agreement the PBM has reached with the manufacturers, says the PBM president. Optum officials said later that the negotiations were still ongoing,