
The drug’s indication for marginal zone lymphoma (MZL) may not be approved until February 2021.

The drug’s indication for marginal zone lymphoma (MZL) may not be approved until February 2021.

Evrysdi is only the second drug approved to treat the rare disease.
Olinvyk is the first new chemical entity in this IV drug class in decades.

The chimeric antigen receptor (CAR) T cell therapy is the first of its kind for mantle cell lymphoma (MCL).

Esketamine (Spravato) can now be used to treat depressive symptoms in adults with major depressive disorder (MDD) with acute suicidal ideation or behavior.

The drug is the first treatment approved for active psoriatic arthritis that selectively inhibits interleukin (IL)-23.

The PBM overcharged for generic drugs, among other offenses, a new lawsuit claims.
The pharma maker is partnering with the nonprofit Civica Rx to help reduce shortages in hospitals.

Five other manufacturers have recalled the popular diabetes medication due to the presence of a the carcinogenic ingredient.

Gilead will present new data on virologic suppression in adults 65 years and older at AIDS 2020: Virtual this week.

Drug helps with heavily-treatment experienced (HTE) adults with multidrug-resistant HIV-1 infection.

Price of the drug to treat hospitalized patients revealed.

Colorectal cancer drug is the first immunotherapy administered as a first-line treatment and without chemotherapy.
These are the top 3 drugs recently approved — or soon to be approved by FDA — to watch in 2020.

Investigational treatment will target marginal zone lymphoma (MZL) and follicular lymphoma (FL).
The agency revoked its emergency use authorization (EUA) for the drug, while warning about a dangerous interaction with remdesivir.

The unique formulation is a dispersible tablet for children.
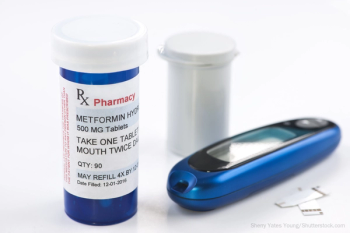
Five pharma markers are recalling the diabetes medication due to the presence of a carcinogenic ingredient.

HHS says supply could run out by the end of this month.

The agency welcomes another option for serious bacterial infections.

Brilinta is already approved to prevent atherothrombotic events in ASC.

Immediately after the FDA said it found elevated levels of the carcinogenic ingredient N-nitrosodimethylamine (NDMA) in certain extended release (ER) metformin products, the first 2 major recalls are underway.

IV artesunate is the only drug available in the U.S. to treat the disease.
FDA authorizes first stand-alone, at-home test, plus other top COVID-19 news stories.
Antigen tests, a new category, have a major advantage and disadvantage over COVID-19 tests already approved.