
The therapy is a modified poliovirus vaccine that not only kills cancer cells but also can trigger long-term immune memory.

The therapy is a modified poliovirus vaccine that not only kills cancer cells but also can trigger long-term immune memory.

The Supreme Court’s dismissal of the latest challenge means patients will have access to lower-cost therapies, an advocacy group says.

The COX-2 inhibitor Anjeso, a faster-acting injectable formulation of meloxicam, reduces opioid use after surgery.

Chances of left ventricular ejection fraction almost disappeared when patient took the ACE-inhibitor lisinopril during treatment with trastuzumab/anthracycline, according to research presented at ASCO.

Immune-related AEs can occur one to two years after treatment, are more common in combination treatments, and can be difficult to manage.

The Alzheimer's Association praises the approval but not the price tag. A Harvard Medical School professor who resigned from an FDA advisory committee says the OK "was probably the worst approval decision in recent U.S. history" in his resignation letter.

Nurtec ODT is the first oral CGRP antagonist approved for migraine prevention, says its maker, Biohaven.

Three commercial plans now cover the corticosteroid Eysuvis.

Today's approval goes against the advice of an outside advisory committee.
Semaglutide injection is a weekly injection for chronic weight management in adults.

Around 49% of the more than 300 healthcare providers, hospital/health system executives, payers, advocacy groups, and academics surveyed by Get the Medications Right™ (GTMRx) Institute said the cost of healthcare is the biggest issue they face.
Prevalent cases of opioid use disorder could total around 1.9 million in 2021 and could cost the U.S. billions to treat, data and analytics firm GlobalData said.
About 2% to 3% of patients with nonsmall cell lung cancer have EGFR exon 20 insertion mutations targeted by newly approved Rybrevant.

OptumRx had identified pegcetacoplan as one of the top 5 drugs in the pipeline this year.
Sanofi and GlaxoSmithKline’s joint COVID-19 vaccine candidate achieved 95% to 100% seroconversion in a Phase 2 study.


The drugs in development include Biogen’s controversial treatment for Alzheimer’s disease and three new drugs for atopic dermatitis.

Teva is making the first generic version of Absorica (isotretinoin), a drug for the treatment of severe acne.
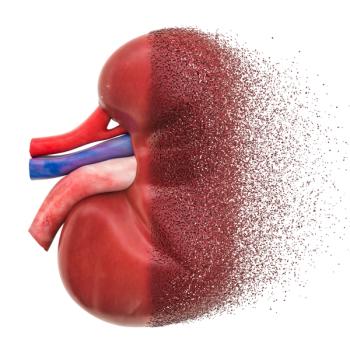
The AstraZeneca drug is the first SGLT2 inhibitor approved for chronic kidney disease regardless of whether the patient has diabetes.
Global drug spending — excluding COVID-19 vaccines — is expected to reach an estimated $1.6 trillion by 2025, a new report says.

The pandemic is expected to have a significant effect on drug costs this year and into 2022, a new report says.

The major depressive disorder (MDD) market is expected to soar to $7.87 billion by 2029.

Opdivo is the first FDA-approved immunotherapy for the first-line treatment of gastric cancer.

The combination monoclonal antibody treatment of bamlanivimab and etesevimab still OK.

The new approval expands the drug's indication to include treatment in adult patients with unresectable locally advanced or metastatic triple-negative breast cancer.
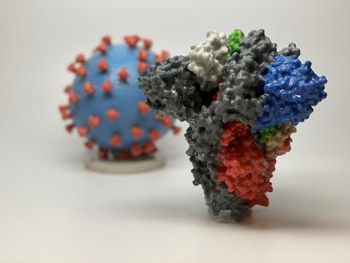
Two common antidepressant and antifungal drugs were very effective at inhibiting SARS-CoV-2, the virus that causes COVID-19, according to a new study.