
The PBM association also appointed Brendan Buck chief communications officer. Both Marin and Buck begin Jan. 20, 2026.

The PBM association also appointed Brendan Buck chief communications officer. Both Marin and Buck begin Jan. 20, 2026.

Drug and hospital prices are top employer healthcare concerns as costs rise, but some employers are unable to access their data, finds a survey by the National Alliance of Healthcare Purchaser Coalitions.
Liraglutide, a generic of Saxenda, is approved to treat adults and adolescents who are obese or overweight.

Some vials in two lots of the antibiotic cefazolin are labeled as being penicillin G potassium, which if used could lead to suboptimal outcomes, adverse events, drug interactions and delayed recovery.

The complete response letter did not identify any issues with the safety or efficacy of Eylea HD, Regeneron officials said.

The FDA has seized several hundred units of counterfeit Ozempic 1 mg

Steqeyma is one of seven Stelara biosimilars that have been approved by the FDA.

Celltrion’s Omlyclo is interchangeable with Xolair to treat the same conditions: asthma, chronic rhinosinusitis, food allergy and chronic spontaneous urticaria.
Regulators have set a goal of Aug. 12, 2025, for brensocatib to treat patients with non-cystic fibrosis bronchiectasis, a chronic lung disease.
Regulatory officials have identified 82 cases worldwide from the FDA Adverse Event Reporting System of anaphylaxis associated with the use of Coxapone and the generic Glatopa that have resulted in hospitalization or death.

The target action date for Rexulti in combination with sertraline to treat patients with post-traumatic stress disorder, originally planned for Feb. 8, 2025, will be delayed.
Kerenida is currently approved to slow the progression of chronic kidney disease associated with type 2 diabetes.

Regulators say, however, the benefits of vaccination with Abrysvo and Arexvy in preventing respiratory syncytial virus continue to outweigh their risks.
FDA officials have set a review date of June 30, 2025, to review avutometinib in combination with defactinib to treat patients with recurrent low-grade serous ovarian cancer.
The boxed warning follows a safety warning the FDA issued in September about the risk of liver injury from the use of Veozah to treat hot flashes.

Attruby (acoramidis) is a small molecule approved to treat adults with transthyretin amyloid cardiomyopathy. It will have a list price of $18,759 for a month’s supply.
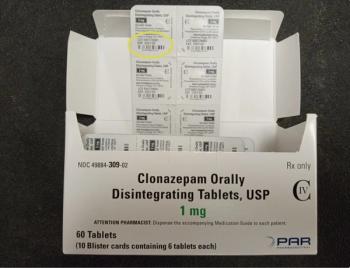
A total of 17 lots are now part of the recall of Clonazepam because some cartons have the wrong strength on the label.
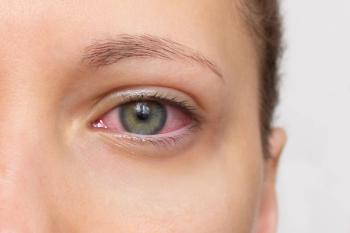
The FDA assigned a Prescription Drug User Fee Act (PDUFA) date of April 2, 2025, for reproxalap for patients with dry eye disease. Reproxalap also is being tested in allergic conjunctivitis.
The FDA has provided a date of April 18, 2025, to review the application for Dupixent to treat patients with chronic spontaneous urticaria, an inflammatory skin disease.
After feedback from the FDA, the companies have voluntarily withdrawn the previous biologics licensing application for datopotamab deruxtecan for patients with advanced or metastatic nonsquamous non-small cell lung cancer.
Aucatzyl is a CAR T-cell therapy that targets CD19 and has been designed to minimize excessive activation of the programmed T cells. The wholesale acquisition cost is $525,000
Regulators are also reviewing Teva’s application for its Prolia biosimilar candidate. The FDA’s decisions are expected mid-year 2025.

PTC Therapeutics' NDA resubmission of Translarna was based on data from Study 041 and the long-term STRIDE registry. No PDUFA action date was provided for regulators.

Gavreto used to treat metastatic RET fusion-positive non-small cell lung cancer and RET fusion-positive thyroid cancer.
Zolbetuximab — now with the brand name Vyloy — is a monoclonal antibody to treat patients with advanced gastric and gastroesophageal cancers.
Imuldose will be marketed in the United States by Accord BioPharma and is expected to launch in the first half of 2025.
Itovebi is approved to treat patients with PIK3CA-mutated HR-positive, HER2 negative metastatic breast cancer in combination with Ibrance and fulvestrant. It will have a cost of $22,867 for a 28-day cycle.

The IQVIA analysis finds that the impact of drug price negotiation on patients’ out-of-pocket spending could also be smaller than expected.

This is the sixth indication for Dupixent and the only biologic approved in the United States to treat COPD.
KarXT — now with the brand name Cobenfy— is expected to be available in October with a list price of about $22,500 annually. The drug represents a new way to treat adults with schizophrenia.

Published: July 25th 2024 | Updated:
Published: July 9th 2024 | Updated:
Published: July 8th 2024 | Updated:
Published: July 17th 2024 | Updated:

Published: September 13th 2024 | Updated:

Published: November 25th 2024 | Updated: