Research at the annual meeting of the American Society of Hematology evaluated patient and caregiver perspectives on gene therapies for sickle cell disease, which offer great potential but have had slow uptake.

Research at the annual meeting of the American Society of Hematology evaluated patient and caregiver perspectives on gene therapies for sickle cell disease, which offer great potential but have had slow uptake.
Researchers at Brigham and Women’s Hospital Harvard Medical School looked at clinical trials and compared these biologics to placebos to see how their treatments improve and manage severe asthma.

Researchers have compiled a list of chemicals commonly found in plastics, including benzophenones, chlorinated paraffins and PFAS, known as “forever chemicals,” and they say there might be a connection to breast cancer.

Researchers are investigating the therapy as a treatment for B-cell malignancies, but they might also investigate as a treatment for multiple sclerosis and lupus.

Authorities have named Mangione, 26, as a person of interest in last week’s fatal shooting of United Healthcare CEO Brian Thompson in Manhattan. He was taken into custody at a McDonald’s in Altoona, Pennsylvania around 9 a.m. ET, officials said.

Here’s what you missed this week on Managed Healthcare Executive.

Aerobic exercise, particularly static exercises like yoga and stretching, performed 70 to 90 minutes three times a week over eight to 10 weeks, can significantly improve sleep disorders in menopausal women.
The combination of Columvi, gemcitabine and oxaliplatin is the first CD20xCD3 bispecific antibody to show positive results in a randomized diffuse large B-cell lymphoma phase 3 trial. The FDA’s decision is expected by July 20, 2025.

A systematic review and network meta-analysis examined research on four types of treatment for children with post-traumatic stress disorder. Trauma-focused cognitive behavioral therapy was the clear winner.

Thompson, who was on his way to attend an annual investor conference in Manhattan today, was shot after 6:45 a.m. in a targeted attack, police officials say.

The FDA has accepted a new drug application for aficamten, a new obstructive hypertrophic cardiomyopathy drug. A goal date has been set for Sept. 26, 2025.
Yesintek is the latest Stelara biosimilar to gain FDA approval and it will be available in February 2025.

A new nationwide study led by the Keck School of Medicine of USC aims to examine how type 1 diabetes impacts children's brain development and cognitive function, focusing on diverse participants and paving the way for early interventions and better diabetes management.
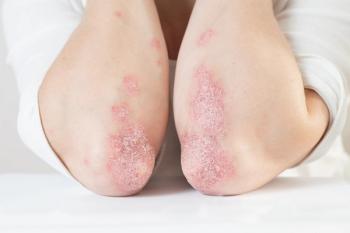
If granted, Tremfya will be approved to treat children ages six and under with severe plaque psoriasis and children ages five and under with juvenile psoriatic arthritis.

Here’s what you missed this week on Managed Healthcare Executive.

Paul Fronstin, director of Health Benefits Research at EBRI, discussed ERISA and trends in employer self-insurance during a conversation with Managed Healthcare Executive recently.

Paul Fronstin, director of Health Benefits Research at EBRI, spoke with MHE about the challenges employers face in incentivizing high-value healthcare while managing costs.

Poor balance and coordination are prominent features of Friedreich’s ataxia (FRDA). Czech researchers found that neuropsychiatric symptoms are also common among patients with FRDA.

Vutrisiran is the generic form of previously approved Amvuttra. The FDA’s target date for the treatment of transthyretin amyloidosis with cardiomyopathy is March 23, 2025.
Imkeldi is a new formulation of imatinib approved as a strawberry-flavored, shelf-stable liquid designed to be more appealing to a wider range of patients, pediatric patients included.

Greg Baker, CEO of AffirmedRx, shared with MHE editors how the company is addressing ongoing challenges in the pharmacy benefit management (PBM) industry, particularly corporate consolidation and transparency issues.
The menopause market is projected to increase at a CAGR of 5.10% between 2025 – 2033, with North America making up 4.9% during this period.

Marty Makary, M.D., M.P.H., as FDA administrator may be the least controversial of Trump's picks for the top healthcare jobs so far.

Here’s what you missed this week on Managed Healthcare Executive.

Patient follow-up 10 years after hormone replacement therapy treatment revealed there were no long-term negative cognitive effects.

In a recent conversation with Greg Baker, CEO of AffirmedRx, the PBM professional outlined the company’s distinct approach in the PBM industry with MHE editors.

Gene therapies have a lot of potential to improve health outcomes for patients with life-threatening diseases but face a number of barriers that restrict access.
Produced in Chinese hamster ovary cells, Ziihera (zanidatamab-hrii) is the first HER2-targeted bispecific antibody treatment for patients with previously treated, unresectable or metastatic biliary tract cancer.

Kennedy's vaccines may give some Republican senators pause while Oz has an ally in Medicare Advantage plans.
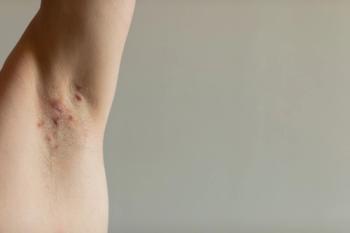
The FDA has approved UCB's Bimzelx for moderate-to-severe hidradenitis suppurativa, offering a new treatment option for this painful autoimmune skin disease.