
At what was supposed to be a hearing to discuss PBM reform, some in Congress veered off into discussions about Medicaid and maternal health, and even Elon Musk.

At what was supposed to be a hearing to discuss PBM reform, some in Congress veered off into discussions about Medicaid and maternal health, and even Elon Musk.

But major cuts to federal for Medicaid are a likely outcome of the $1.5-$2 trillion in spending cuts over the next 10 years that the Republican-backed budget resolution calls for.

Unlike some other spending cuts, Medicaid cuts will have direct impact on people who voted for Republicans in November 2024, according to Leanne Berge, health plan leader

Although the importance of ongoing education is known for patients, less than half of those with diabetes receive formal instruction on managing their condition.

After receiving the Day 74 letter, which outlines the FDA’s initial assessment of the application, the agency accepted Epioxa for review for efficacy and safety and plans to complete the process by October 20, 2025 under the Prescription Drug User Fee Act (PDUFA).

Here’s what you missed this week on Managed Healthcare Executive.

Obesity rates have spiked over the past few decades, displaying itself a significant crisis.

Conversations around menopause symptoms are becoming more common, but there is such a thing as potentially calling too much attention, especially in the workplace, according to Monica Christmas, M.D., director of the menopause program at the University of Chicago Medicine and the Center for Women’s Integrated Health.

Gaps in funding caused by the potential $5 billion budget cut to the National Institutes of Health would be “almost impossible” to fill, according to Aaron J. Kowalski, Ph.D., CEO of Breakthrough T1D.

In this role, Virtell will oversee the strategy, daily operations and expansion of Cigna Healthcare’s Supplemental Health division, which helps customers manage out-of-pocket expenses from serious illnesses or accidents.

Bias and stigma are to blame for the way women’s health research has fallen behind, according to Monica Christmas, M.D., director of UChicago Medicine’s menopause program and the Center for Women's Integrated Health and Valentina Sartori, Ph.D., a partner in McKinsey & Company’s Life Sciences Practice, and affiliated leader of the McKinsey Health Institute.

Funding will be cut to $10 million, which is a nearly 90% decrease from 2024’s budget of $98 million.

Lenacapavir is a twice-yearly injectable medication designed to be used as pre-exposure prophylaxis (PrEP). The FDA is giving this drug a priority review and expects to make a decision by June 19, 2025, according to a release.

Aaron J. Kowalski, Ph.D., CEO of Breakthrough T1D, spoke with Managed Healthcare Executive, about the possible impacts of the NIH’s cuts to indirect research funding.


Here’s what you missed this week on Managed Healthcare Executive.

Inhalon plans to begin a human challenge for their inhaled antibody therapy study in 2026.
Gomekli is the second ever FDA-approved treatment for rare tumor disease, NF1-PN and the first to be approved for both adult and pediatric patients.

The opinions of Drew Altman, Mark McClellan and Don Berwick
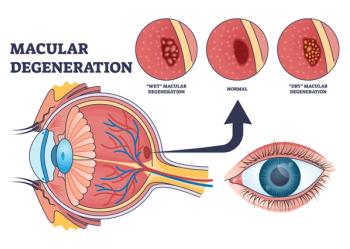
The updated label allows physicians to prescribe Izervay without a limitation on duration for patients with geographic atrophy secondary to age-related macular degeneration.

The group released a statement today expressing that they are eager to work with Robert F. Kennedy Jr. to eradicate HIV when steps into his role as the Secretary of Health and Human Services.

Positive HIV status as well as socioeconomic disadvantages, not breastfeeding, are associated with infant length and birth weight, according to the results of a new South African study.

Those voting to support Robert F. Kennedy Jr as Secretary of HHS include Louisiana Republican Sen. Bill Cassidy. Mitch McConnell, a Republican from Kentucky, however, voted no.

The second part of our conversation with Craig Burton, MBA, executive director of the Biosimilars Council, a trade and lobbying group for the biosimilars industry.

Menopausal patients are most interested in learning more about non-hormonal treatments to address their symptoms, according to the results of an international survey led by the Menopause Priority Setting Partnership (MAPS). Monica Christmas, M.D., director of the menopause program at the University of Chicago Medicine and the Center for Women’s Integrated Health, discussed the survey details with Managed Healthcare Executive.

CVS Health’s healthcare benefits segment, which includes Aetna, had medical benefit ratio of 92.5% in 2024, because of higher utilization, an unfavorable impact of Medicare Advantage star ratings payment and higher levels of care in Medicaid.

We spoke recently with Craig Burton, MBA, executive director of the Biosimilars Council, a trade and lobbying group for the biosimilars industry.
The respiratory tract’s microbial microflora plays a significant role in determining the severity of RSV infections and the development of these long-term conditions.


Judi Health manages medical and pharmacy claims on the same platform to cut administrative cost.