
Here's how one federally qualified health center in southcentral Pennsylvania is tackling the opioid crisis in its community.

Here's how one federally qualified health center in southcentral Pennsylvania is tackling the opioid crisis in its community.

HealthKonnect, a subsidiary of Chinese insurer Ping An Insurance Group developed a personal health-risk prediction model that has the ability to predict at individual patient level future healthcare risks measured by total medical costs. Here’s how.

Attending the AMCP Managed Care and Specialty Pharmacy Annual Meeting in Boston April 23 to April 26? Here are our top session recommendations.

A study from the Intermountain Medical Center Heart Institute has shocking findings about certain blood pressure medications and mortality outcomes.

After US Surgeon General Jerome M. Adams, MD, MPH, urged more Americans to carry naloxone to reverse opioid overdoses, organizations and medical experts praised the decision.

A new Private Exchange Research Council study shows why decision support and educational tools are necessary to help employees navigate the choice in benefits.

Healthcare systems stand to lose $6 million if burned out physicians choose to resign or work elsewhere. Here are some ways to curb burnout and its associated costs.

A new deal between pharma giants AbbVie and Samsung Bioepis is designed to postpone competition against a biosimilar to Humira until 2023.

HealthPartners’ vice president and associate medical director shares how the integrated organization is looking beyond traditional approaches to curb diabetes.

In the shift to value care, value-based purchasing guidelines reward positive quality metrics with bonuses and levy fines and assessments on institutions that are unable to demonstrate quality of care. Often however, it’s not the care itself that’s at fault, but the reporting. Here’s an approach to capturing those crucial metrics.

Diabetes drug spending is on the rise, but there are ways to control it.
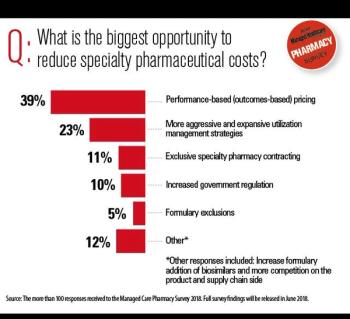
Experts weigh in on the spending outlook for these high-cost conditions.

Gene therapy is leaving its mark, while payers contemplate payment models, value, and efficacy issues.

Capturing the power of big data is key to patient care strategies that reduce costs, improve outcomes and enhance patient experience.

Here’s how new technologies and technology partnerships could change the landscape of diabetes care.

A new study shows that ACA tobacco rating rules aren't enforced in employer plans.

ECRI Institute names diagnostic errors the number one concern on its Top 10 Patient Safety Concerns for Healthcare Organizations. Here’s how technology tools can help.

A new study has surprising findings about how seniors feel about healthcare and technology.

A new Oliver Wyman study reveals costly findings about Part D beneficiaries enrolled in plans without preferred pharmacy networks.

A new study from Sage Growth Partners has shocking findings about EHRs and value-based care.

We know you’re pressed for time, so we’re here to help. Listen to this two-minute podcast for critical takeaways from three of our top articles.

A UCLA study shows that the Great Recession intensified patients’ cardiovascular risk factors.

FDA okayed the marketing of a new continuous glucose monitoring system for diabetics-the first to be used as part of an integrated system with other compatible medical devices and electronic interfaces.

There are a host of causes for a patient with chronic obstructive pulmonary disease (COPD) to be hospitalized, ranging from pneumonia to infection.

PBMs are in a unique position to leverage three critical tools to protect patients from unnecessary healthcare costs.

Experts weigh in on a recent lawsuit against PBMs, whether similar lawsuits will crop up, and the likely outcomes.

A new Symantec survey has surprising findings about risk assessment as a driver in cybersecurity investments.

FDA’s approval of a new combination treatment for patients with classical Hodgkin lymphoma (cHL) is the first new advanced treatment for the disease in 40 years

Due to problems with compounded medications- including the 2012 fungal meningitis outbreak that led to 64 deaths-FDA is releasing a draft guidance that will determine which bulk drug substances outsourcing facilities can use to compound drugs.