The supply interruption is the result of a combination of factors, including significant increases in demand, an increase in syphilis infection rates, as well as competitive shortages.

The supply interruption is the result of a combination of factors, including significant increases in demand, an increase in syphilis infection rates, as well as competitive shortages.

If passed, PBMs would not be able to charge fees that are connected to the price of a drug, discounts, or rebates.

Ziprasidone is used to treat patients with schizophrenia and bipolar disorder. Taking dronabinol instead may lead to worsening of mental illness symptoms.
Ozempic, Wegovy and other GLP-1 drugs present a challenge to payers in terms of costs and outcomes, finds PSG’s new Drug Benefit Design Report.
SmithRx will offer Yusimry through Mark Cuban’s online pharmacy for $569.27, and members will be able to apply their insurance benefits.

Linzess is first FDA-approved prescription therapy for functional constipation in children 6 to 17 years of age.
If approved, nirsevimab would be the first immunization specifically to protect infants through their first RSV season. The Prescription Drug User Fee Act date is in the third quarter of 2023.

More than 90% of cancer centers have experienced shortages of critical drugs. Erin R. Fox, Pharm.D., University of Utah Health, talks about why these shortages are happening and efforts that are being made to address them.
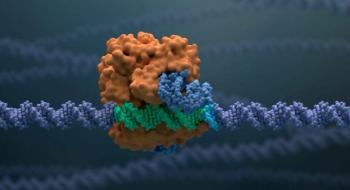
Exagamglogene autotemcel is being evaluated for patients with sickle cell disease and beta thalassemia, in which a patient’s own hematopoietic stem cells are edited to produce high levels of fetal hemoglobin in red blood cells.

Vevye (previously CyclASol) is a water-free, preservative-free solution, which allows for improved bioavailability and better efficacy on the target tissue.

Renee Rayburg talks about how paying for specialty drugs continues to be a top concern for both health plans and employers.

The FDA has received adverse event reports after patients have used compounded semaglutide that contains salt formulations, which are different active ingredients than that used in the agency-approved drugs for diabetes and weight loss.
The median annual cost for new oncology medicines launched in 2022 was $260,000, up from $63,534 10 years ago, an IQVIA report finds.
The FDA has assigned an action date of Sept. 23, 2023. If approved, this would be the first frontline treatment advancement in decades for patients with advanced or recurrent endometrial cancer.

In an analysis, researchers determined that Inpefa is cost-effective at commonly accepted willingness-to-pay thresholds in treating patients with heart failure who have diabetes.

Prevymis was approved to prevent CMV infection in adults after an allogeneic hematopoietic stem cell transplant in 2017.

The combination of Lynparza and abiraterone reduced the risk of disease progression or death by 76% vs. abiraterone alone in patients with BRCA-mutated metastatic castration-resistant prostate cancer.
The drugs must have full “traditional” FDA approval and patients need to be enrolled in a registry that collects post-approval data efficacy and safety when the drugs are used in clinical practice.
Coherus will launch Yusimry in July with a list price of $995 per carton (2 x 40 mg/0.8 mL autoinjectors); “the lowest price announced to date of any adalimumab offering in the United States,” the company said.
Miebo is the first and only prescription eye drop approved for dry eye disease (DED) that directly targets tear evaporation.
Lifileucel is a polyclonal tumor infiltrating lymphocyte (TIL) therapy designed for patients with advanced melanoma who have experienced progression after previous treatment with anti-PD-1/L1 therapy and targeted therapy.
Inpefa (sotagliflozin) is in the SGLT inhibitor class recommended as first-line treatment for heart failure by the American Heart Association and other groups. Jardiance (empaglifozin), a competitor in that class, has a list price of $570.48 for a month's supply.

The frequency of drug manufacturer coupon utilization is associated with more market competition—but not patients’ out-of-pocket costs.

More than 11.6 million treatment courses of the medication have been prescribed in the U.S. to date.

AbbVie and Genmab have set a price for newly approved Epkinly (epcoritamab-bysp).

The FDA recently approved Brixadi to treat moderate to severe opioid use disorder in patients who have already started treatment with a transmucosal form of buprenorphine, and have approved Opvee as an emergency treatment to reverse known or suspected opioid overdose in people ages 12 years and older.