Welireg is now also indicated for patients whose renal cell carcinoma has progressed after treatment with PD-1 or PD-L1 inhibitor and a TKI therapy.

Welireg is now also indicated for patients whose renal cell carcinoma has progressed after treatment with PD-1 or PD-L1 inhibitor and a TKI therapy.

Zurzuvae has a list price of $15,900 for a 14-day course of treatment. No coverage information has been released.
The FDA has set a target date of June 12, 2024, for tarlatamab to treat adult patients with advanced small cell lung cancer. If approved, it would be the first bispecific antibody for a solid tumor.

Drug prices and the practices of PBMs are among the concerns expressed by employers who responded to the Pulse of the Purchaser survey by National Alliance of Healthcare Purchaser Coalitions.

The application for midomafetamine is the first NDA submission for any psychedelic-assisted therapy.

Optum Rx standard formularies currently include the weight loss drugs Wegovy and Saxenda, and the PBM is evaluating Zepbound.
Marstacimab is being reviewed to prevent or reduce the frequency of bleeding episodes in people with hemophilia A or B. The FDA has set an action date in the first quarter of 2024.

Eight of 10 drugs reviewed by Institute for Clinical and Economic Review were not supported by clinical evidence.
Cipla is recalling one lot of vigabatrin, which is used to treat seizures.
The FDA has approved the gene therapies Lyfgenia and Casgevy to treat patients with sickle cell disease. Casgevy is the first FDA-approved gene therapy to use the CRISPR gene editing technology.
If approved, elafibranor would be a second-line treatment for patients with primary biliary cholangitis. The Prescription Drug User Fee Act action date is June 10, 2024.

IQVIA’s Michael Kleinrock and experts at RxBenefits reveal during webinar top pharmacy trends going into 2024.

Augtyro, approved last month, launched with a month wholesale acquisition $29,000 for patients with ROS1-positive non-small cell lung cancer.
Researchers suggest a head-to-head comparative effectiveness study of Ocrevus and Rituxan/biosimilars in patients with multiple sclerosis is needed for payers to negotiate prices for these therapies.
Fabhalta was approved to treat patients with paroxysmal nocturnal hemoglobinuria and will have a wholesale acquisition cost of $550,000 per year.

CarelonRx says it will be able provide optimal support for members because, as a PBM, it as able to communicate directly with members’ providers.
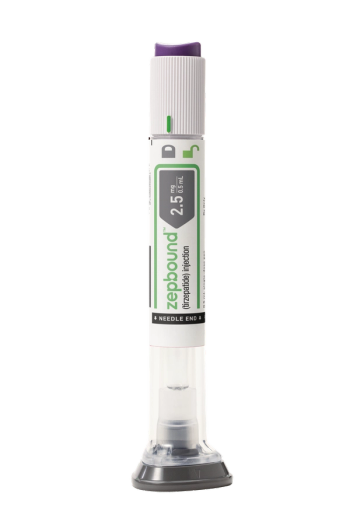
Zepbound, which was approved last month to treat obesity, is now available in U.S. pharmacies.

Shortages are growing because of disruption caused natural disasters and ingredient supply issues, as well as increased demand for certain drugs.

Jaypirca is also approved to treat mantle cell lymphoma. It has a list price of $21,000 for a 30-day supply.

Twice-daily danuglipron was being studied in a phase 2 study in adults with obesity. Pfizer is now conducting a pharmacokinetic study for a once-daily formulation.

A new survey finds that more people are using semaglutide and other GLP-1 therapies to lose less than 15 pounds.

Although biosimilars have already generated savings for Medicare Part B programs and beneficiaries, opportunities for substantial reductions in spending remain, according to a report from the HHS.

Adzynma is a recombinant protein designed to replace the deficient ADAMTS13 enzyme to treat patients with the rare hereditary blood clotting disorder congenital thrombotic thrombocytopenic purpura

The FDA has set a date of July 07, 2024, for Zorvye 0.15% for adults and children six and up with the chronic skin disease atopic dermatitis.

Regulators have found that medications containing levetiracetam and clobazam have led to drug reaction with eosinophilia and systemic symptoms (DRESS), a rare and severe drug allergy.