
Medexus is working with Ethypharm to generate the data needed to support an abbreviated new drug application for triamcinolone hexacetonide.

Medexus is working with Ethypharm to generate the data needed to support an abbreviated new drug application for triamcinolone hexacetonide.

The topical anesthetic was found to be “super potent,” which could lead to systemic toxicity and central nervous system and cardiovascular reactions.
The new boxed warning for Xeljanz, Olumiant, and Rinvoq includes the risk of cardiovascular adverse events and cancer.
Glass has been detected in the two lots of Veklury, which is used to treat patients who are hospitalized with COVID-19.

A temperature change during shipping could impact the product’s effectiveness. Enoxaparin is used to prevent deep vein thrombosis.
The warnings section of Inrebic, a JAK inhibitor to treat a bone marrow cancer, now includes information about the cardiovascular risk associated with Xeljanz, another JAK inhibitor to treat arthritis.

New requirements resulted in long call wait times, which has led to patient access issues.

The incidence of brain swelling was highest in the group of patients who received the highest doses of Aduhelm, as well as the group of patients who are carriers of the APoE gene.

The antiseizure medication affected by the recall could have sterility issues.


Equipment and process issues could lead to a lack of sterility.


The safety labeling for the beta interferons that are used to treat multiple sclerosis have been updated to include warnings about injection site reactions.

The studies conducted to support Verzenio’s most recent approval for the treatment of high-risk early breast cancer provided additional safety data.



The label now includes new data from the trial that supported Cyltezo’s application for interchangeability with Humira.

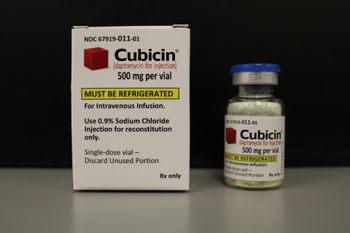
Glass was found in one vial of Cubicin, which treats patients with Staph infections of the skin and blood.


The product contained N-nitrosoirbesartan impurity, a probable human carcinogen.
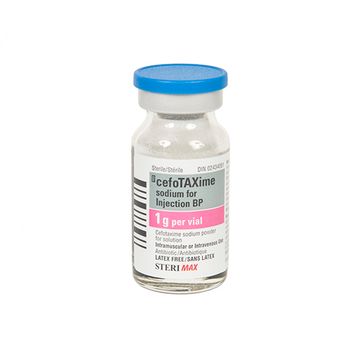
Hikma, which makes the broad-spectrum antibiotic, has experienced manufacturing delays.

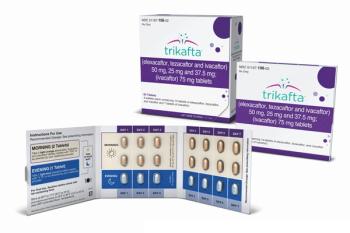
The labeling of Trikafta, which treats cystic fibrosis, has been updated to warn about the possibility of liver failure that could lead to the need for transplantation.