
Early efficacy data from a phase 2a safety trial on the subject was presented during an oral session at the American Thoracic Society 2024 International Conference in May.

New Blood Biomarker Study Identifies Distinct Endotypes in Pulmonary Fibrosis Patients

Early efficacy data from a phase 2a safety trial on the subject was presented during an oral session at the American Thoracic Society 2024 International Conference in May.

A new study published May 2024 in Scientific Reports examined the changes in morphology and antibacterial activity in four honey varieties from Hungary matched against respiratory pathogens.

A new study published April 2024 in the JAMA Network looked to establish a link between burn pits and higher respiratory and cardiovascular disease in veterans, responding to widespread concern from veterans.
In 2022 alone, an estimated 10.6 million new TB cases were reported, mainly in low-income regions, highlighting the urgent need for further efforts in TB prevention and control.

Although chronic respiratory diseases remain the third leading cause of mortality, they have received less attention from the global community.
Rapidly progressing interstitial lung disease in anti-melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis (DM) is known for its high death rate because it worsens quickly and causes breathing difficulties within three months of initial lung symptoms. However, there isn't much information about the timing between the diagnosis of ILD and MDA5 antibody-positive DM because most research focuses on either the frequency or the death rates rather than the timing of onset.


A new study points to disparities for patients in respiratory failure, which can have dire consequences. The lead researchers talk about the implications.
Airsupra is an anti-inflammatory rescue medication that can treat the symptoms of asthma while helping to prevent an attack.
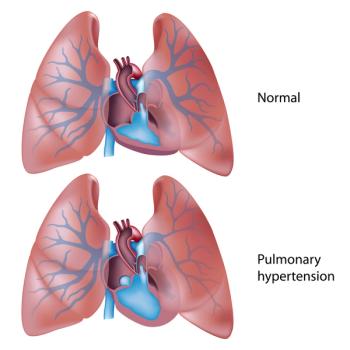
Sotatercept is a first-in-class therapy to treat the rare disease pulmonary arterial hypertension. The FDA has assigned a target action date of March 26, 2024.
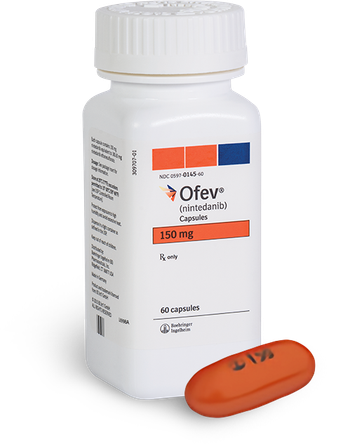
If approved, Ofev would be the first approved treatment for children and adolescents with fibrosing interstitial lung disease. A decision is expected in the fourth quarter of this year.

Tezspire is a biologic that specifically targets the inflammation associated with asthma.

Airsupra is the first rescue medication to manage both the symptoms and the inflammation associated with asthma.
Nirsevimab is a single-dose, long-acting antibody to protect infants from respiratory syncytial virus (RSV) lower respiratory tract infections. The FDA action date is in the third quarter of 2023.
If approved the vaccine, RSVpreF, would be available for people 60 years of age and older. The Prescription Drug User Fee Act goal date for a decision is in May 2023.

With this approval, about 300 children between 12 months and 24 months will be eligible for treatment with Orkambi.

Coherus’ Cimerli has been approved to be interchangeable for all five indications, including age-related macular degeneration and diabetic retinopathy. It will be available in early October 2022.

Two studies conducted by AllianceRx Walgreens Prime call attention to the importance of adherence for reducing risk of negative health outcomes and financial costs.

In high-deductible plans, patients had the highest out-of-pockets costs and abandoned and discontinued therapy at greater rates than other benefit designs.

Tyvaso DPI is a dry inhalation powder and new inhalation device to treat patients with pulmonary arterial hypertension, a life-threatening disease. It is expected to be available in June 2022.

Called Breyna, this drug-device combination generic product is a metered-dose inhaler, which contains both budesonide and formoterol.

The FDA has asked for clinical data for Fasenra, which is being reviewed as a treatment for chronic rhinosinusitis with nasal polyps.
The composition of the microbiota of the airways of people with chronic obstructive pulmonary disorder explains varying degrees of inflammation, according to a review article published recently in the Annals of Medicine.

University of Buffalo researchers found that men with low incomes were at greater risk of hospital readmission.