
Even small payments can influence ophthalmologists and optometrists to prescribe branded therapies to treat patients with glaucoma.

Even small payments can influence ophthalmologists and optometrists to prescribe branded therapies to treat patients with glaucoma.

Patients who received the chemotherapies paclitaxel and docetaxel were at an increased risk of adverse events of the eye.

Novaliq’s CyclASol uses a new technology that allows cyclosporine to be soluble without water or preservatives.

A meta-analysis has found that depression and anxiety are significantly correlated with dry eye disease symptoms but not to dry eye disease signs.
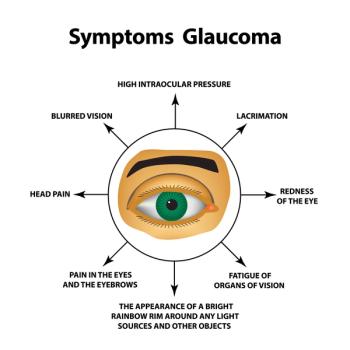
A literature review finds that glaucoma care changed for the better during the COVID-19 pandemic.

Vabysmo is the first injectable bispecific antibody to treat wet age-related macular degeneration and diabetic macular edema. Some recent data suggests that the injections can be administered less often than first indicated with no loss of efficacy.

Coherus’ Cimerli has been approved to be interchangeable for all five indications, including age-related macular degeneration and diabetic retinopathy. It will be available in early October 2022.
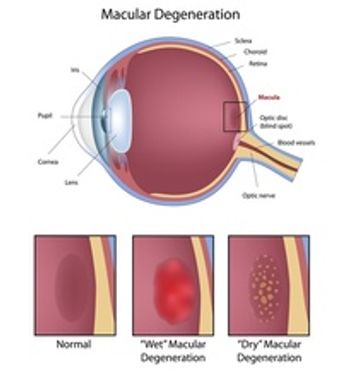
Pegcetacoplan is a targeted therapy to treat patients with age-related macular degeneration. The Prescription Drug User Fee Act (PDUFA) target action date is Nov. 26, 2022.
Those with age-related macular degeneration like the port delivery system for ranibizumab because it requires fewer treatments and there is less discomfort.

Reproxalap is a potential new therapy for dry eye disease with a novel mechanism of action.
NOV03 (perfluorohexyloctane) is a first-in-class eye drop with a novel mechanism of action. If approved it will be the first to address signs and symptoms of dry eye disease.

In a study, both common and rare HMGCR variants were associated with an increased risk for cataract.
Regeneron is seeking an additional dosing regimen with a longer-term interval between doses. The PDUFA date is Feb. 28, 2023.
Xipere became commercially available in March 2022. It is the first therapy for patients with macular edema that provides a targeted delivery to the retina.
A biomarker used in kidney disease screening has potential to identify people with sight-threatening diabetic retinopathy.

Perfluorohexyloctane prevents excessive tear evaporation and has the ability to restores tear film balance. A new drug application is expected to be filed this year.
Byooviz, the first FDA approved ophthalmology biosimilar for macular degeneration, will be priced 40% lower than the reference product. It will be commercially available on July 1, 2022.

If approved, Orasis’ low-dose pilocarpine would be the second product that improves presbyopia, which is the age-related loss of clear up-close vision. The company plans to submit an NDA in the second half of the year.

Briana Contreras, editor of Managed Healthcare Executive, spoke with Nancy Lurker, CEO and president of EyePoint Pharmaceuticals. Nancy shared a bit about EyePoint and how the organization’s innovative therapies are addressing patient needs through eye care, and most importantly, she addressed C-Suite positions like the CEO role. Nancy shared advice for those seeking to reach the CEO level, especially toward women in healthcare and other roles, and what it takes to run a biopharma company.

Phase 3 trials show CSF-1, a low-dose pilocarpine, met clinical endpoints for improving presbyopia, or age-related blurry vision.

Xipere is a targeted treatment that is delivered via an injection to the back of the eye to treat macular edema associated with uveitis, a form of eye inflammation.

Cystaran is a topical ophthalmic therapy that is used to treat a rare genetic disorder.

This expands commercial coverage to 118 million lives and Medicare coverage to 7.1 million lives.

The use of digital devices has surged significantly as people spend more time at home, with Americans logging an average of 13 hours per day watching screens. That compares to between seven and 10 hours per day before the COVID-19 pandemic started, with the increase in screen time likely contributing to more exposure to blue light.

Vuity, developed by Allergan, is a once-daily eye drop to treat presbyopia.