
Improvement of vitiligo repigmentation in patients can result in higher Vitiligo Noticeability Scale values when assessed from patient-reported outcome measures (PROMs) versus traditional investigator-assessed studies.

Improvement of vitiligo repigmentation in patients can result in higher Vitiligo Noticeability Scale values when assessed from patient-reported outcome measures (PROMs) versus traditional investigator-assessed studies.
Zoryve is a next-generation topical PDE4 inhibitor to treat adults and adolescents with plaque psoriasis.

Zoryve, the first topical PDE4 inhibitor approved to treat patients with plaque psoriasis, will be available by mid-August.
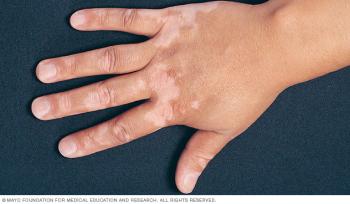
Opzelura is first therapy approved to treat patients with vitiligo, a disease that causes the loss of skin color.
Piclidenoson is a first-in-class, A3 adenosine receptor agonist small molecule that inhibits the inflammatory cytokines interleukin 17 and 23.

Olumiant is the first systemic therapy available to treat alopecia.
Dupixent is the first biologic medicine approved to treat moderate-to-severe atopic dermatitis from infancy through adulthood.

If approved, Dupixent would be the first and only medicine in the United States specifically indicated to treat prurigo nodularis, or persistent itch with thick skin lesions. The PDUFA target action date for the FDA decision is Sept. 30, 2022.
Dr Bhatia concludes our discussion by outlining what factors physicians should be cognizant of when prescribing therapies to patients with AD.

A dermatology expert highlights the role JAK inhibitors play in AD therapy, from mechanism of action to ruxolitinib and the TrUE AD1 and -2 trials.
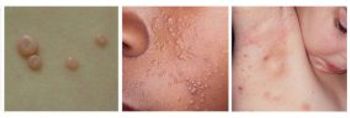
Deficiencies at the contract manufacturing company are, for the second time, holding up approval of VP-102 to treat molluscum contagiosum, a mild skin infection.

Vtama is approved to treat adults with mild, moderate, and severe psoriasis. It is expected to be available in the first week of June 2022.

Dr Neal Bhatia explains the available treatment options for patients with AD, including their relation to guideline recommendations.

Neal Bhatia, MD, provides an overview of atopic dermatitis (AD) and the diagnostic process, as well as how its severity impacts the patient.
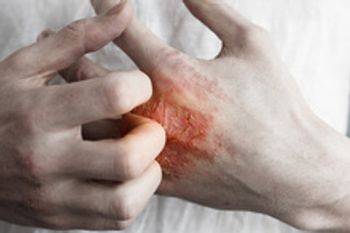
Bimekizumab, now called Bimzelx, is a monoclonal antibody being reviewed as treatment for adults with moderate-to-severe plaque psoriasis.
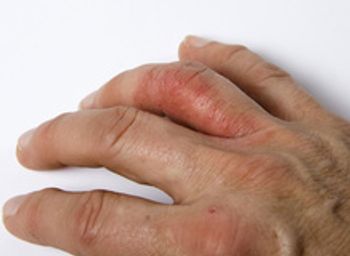
The study aims to generate data related to Tremfya in people of color who have plaque psoriasis.

Opzelura is being reviewed as a treatment for vitiligo, a disease that causes the loss of skin color. The new PDUFA date is July 18, 2022.
Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.

The FDA has assigned a Prescription Drug User Fee Act goal date of Sept. 10, 2022, for deucravacitinib. If approved, it would be the first TYK2 inhibitor approved for the treatment of any disease.

COVID-19 travel restrictions have hindered the FDA’s ability to inspect European manufacturing facilities for bimekizumab, which is under review for the treatment of patients with psoriasis.

The tools for formulary management for atopic dermatitis need flexibility to take into account quality-of-life issues and patient access.

Opzelura is the first and only topical formulation of a JAK inhibitor approved in the United States.
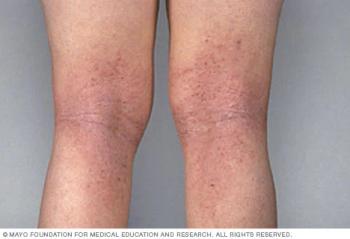
ICER’s policy recommendations suggest payers establish step therapy with less expensive systemic agents to allow patients and clinicians to choose from multiple options.

The single-dose antibiotic with a one-hour infusion is approved to treat MRSA and other skin infections.

Genetic testing is playing a growing role in diagnosing and treating diseases, particularly when it comes to certain cancers and rheumatic conditions.