Biosimilars
Latest News
Latest Videos

CME Content
More News

Discussion on how payers and providers collaborate to enhance patient care and cost management. The upcoming entry of biosimilars post-2023 and their potential influence is also covered.

Challenges with biosimilars and unbranded biologics are discussed and how can they be improved.

An overview of how benefit channels, such as pharmacy benefit and medical benefit, differ and how it impacts utilization of biosimilars and unbranded biologics.

How an open marketplace impacts future competition, pricing transparency, and formulary management.

Savings and incentives associated with the utilization of biosimilars and unbranded biologics, including a look at how it impacts formulary access.

The role of evidence-based recommendations around biosimilars and unbranded biologics to increase patient care, plus how payers and providers evaluate biosimilars through clinical data across multiple indications.

Definitions of originator biologics, biosimilars, and unbranded biologics, including their lifespan in the marketplace.

Amgen’s Amjevita was already on the pharmacy benefit manager’s National Preferred Flex Formulary.
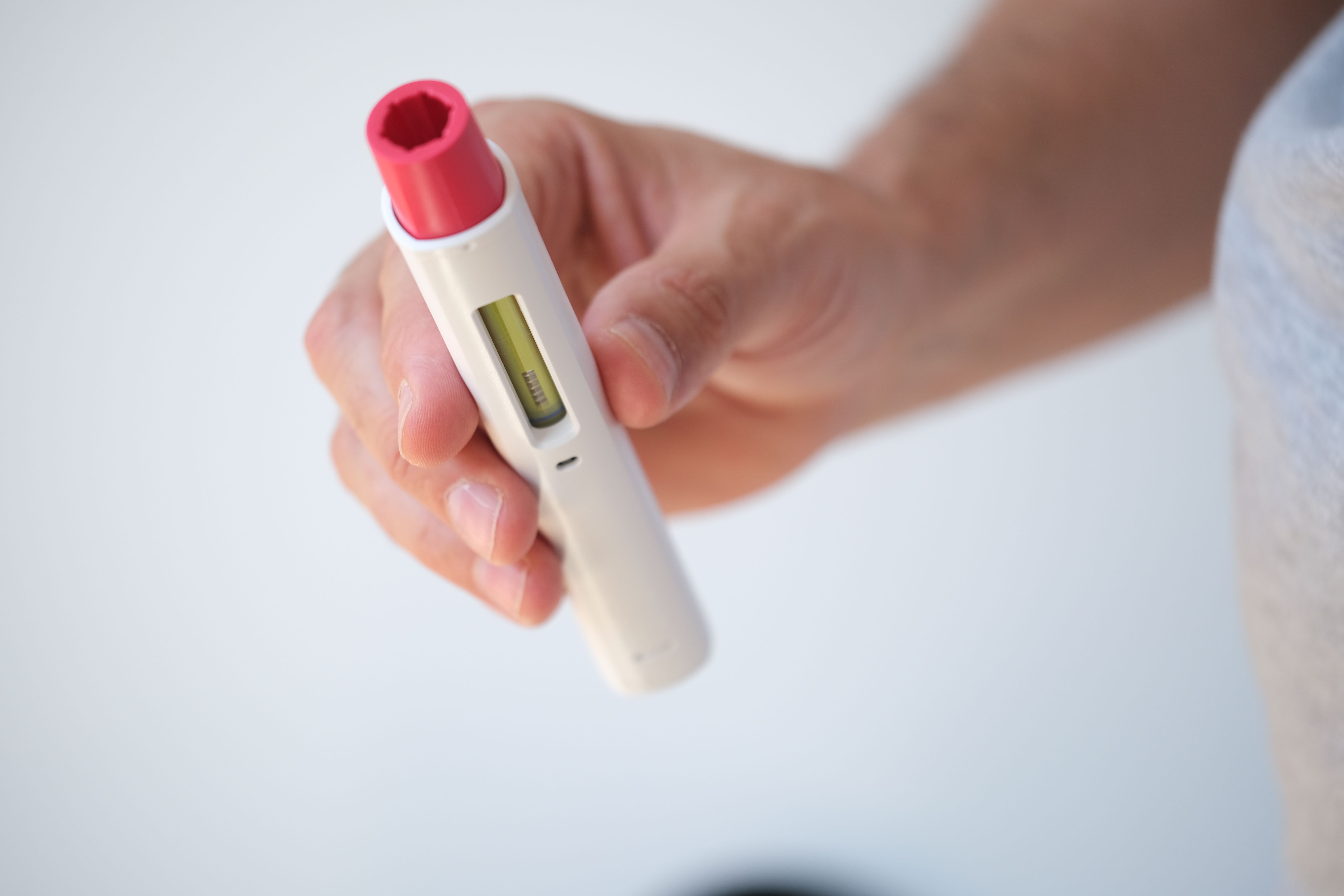
7 of them launched during the first several days of July, joining Amgen's Amjevita in the suddenly crowded market of Humira competitors. Pfizer’s Abrilada is the only FDA-approved Humira biosimilar that hasn’t launched.

Boehringer Ingelheim is pricing its biosimilar 5% to 7% below AbbVie’s top selling drug.

Sandoz will now launch both high-concentration and low-concentration versions of Hyrimoz (adalimumab-adaz) in July.

Cuban, of "Shark Tank' fame and owner of the Dallas Mavericks, called drugs prices ‘insane’ and used some profanity when talking pharmacy benefit managers at the annual meeting of the Association for Accessible Medicines yesterday. Cuban has co-founded an online pharmacy that he says will make drug prices lower and transparent.
As a dozen Humira Biosimilars are set to enter the market this year, it seems to be unlikely that they will drive down drug costs in the future.
Biosimilars started to gain a firm foothold in the market this year and could take off in 2023 with the advent of Humira biosimilars. Our articles about biosimilars were some of the most viewed of the year.

Kareem Karara, Pharm.D., BCPS, CCHP, spoke about unbranded biologics, which are identical the branded products. They are coming on the market amid a growing number of biosimilars.

OptumRx will offer Amjevita and two other biosimilars alongside Humira. OptumRx is the first large PBM to make public its plans for the seven Humira biosimilars that may come on the market next year.
The question of whether it is safe to switch among biosimilars, not just between biosimilars and the reference product, is coming more pertinent as the number of biosimilars on the market increases.
A phase 3 trial sponsored by the biosimilar maker shows that its candidate, temporarily named MYL-1601D, has the same immunogenicity, efficacy and safety levels as NovoLog, Novo Nordisk’s brand-name insulin aspart. Novolog and its authorized generic currently have the U.S. insulin aspart market to themselves.

The review focused on Remicade (infliximab) and Enbrel (etanercept). The annualized discontinuation rate was 21% among patients who had undergone nonmedical switching to biosimilars. However, the rates were similar to those found in some separate analyses of patients on reference biologics.

Several provisions in the law are designed to protect and foster the biosimilar market. But some representatives of the sector see CMS drug price negotiation as a threat to profit margins.

The Inflation Reduction Act puts a $35 cap on monthly out-of-pocket expenses for insulin. The catch: It applies only to people covered by Medicare. Some states, including California, and Civica Rx, the generic drugmaker, are gearing up to make biosimilar versions of brand-name insulin products. The competition could drive down prices.

Disease flareups occurred in seven women who stopped biosimilar treatment and two who continued without stopping, according to findings published in the journal Obstetric Medicine.

Humira may face competition from multiple generics next year. An octet of biologics may see competition this decade.

Metaphors of brewing beer and other concepts may help overcome some of the reluctance to prescribing biosimilars.
But the out-of-pocket costs were 47% to 59% lower for patients treated with the biosimilars Fulphia and Udenyca as primary prophylaxis for febrile neutropenia.